Market Summary
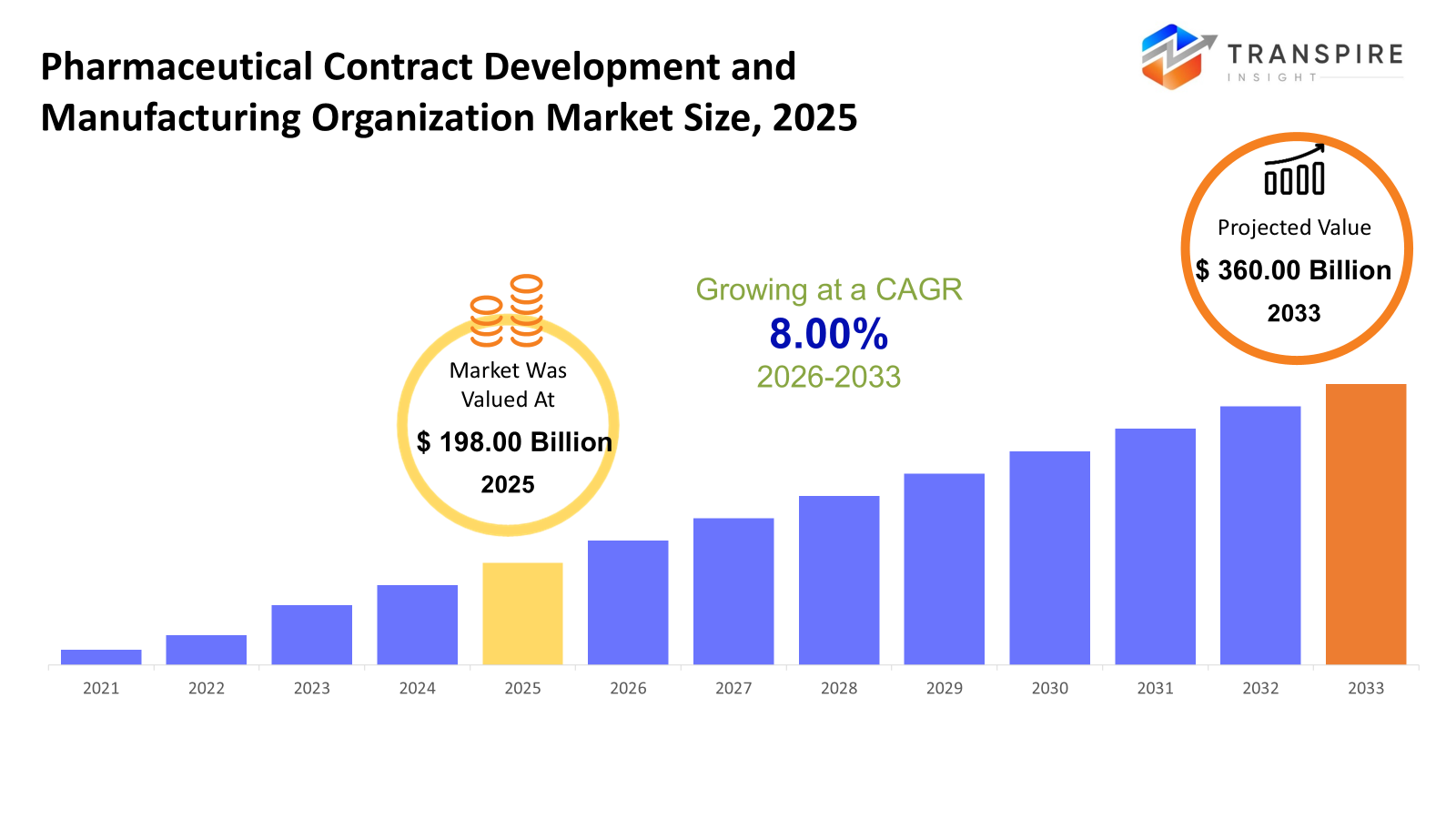
The global Pharmaceutical Contract Development and Manufacturing Organization market size was valued at USD 198.00 billion in 2025 and is projected to reach USD 360.00 billion by 2033, growing at a CAGR of 8.00% from 2026 to 2033. The pharmaceutical Contract Development and Manufacturing Organization market is witnessing strong growth driven by the increased practice of outsourcing pharmaceutical and biotech industry drug development and manufacturing activities. This practice is aimed at optimizing both cost and operational flexibility for those industries. The rising complexity of biologics, a burgeoning clinical pipeline and the need for specialized manufacturing also support the trend of CDMO partnerships. Finally, regulation, speed-to-markets issues, and investment in new modes of therapies add tailwinds for the continued growth of the pharmaceutical CDMO industry.
Market Size & Forecast
- 2025 Market Size: USD 198.00 Billion
- 2033 Projected Market Size: USD 360.00 Billion
- CAGR (2026-2033): 8.00%
- North America: Largest Market in 2026
- Asia Pacific: Fastest Growing Market
To learn more about this report, Download Free Sample Report
Key Market Trends Analysis
- North America continues to show a growth trend based on strong pharmaceutical innovation, advanced infrastructure for biologics manufacturing and outsourcing collaboration between pharmaceutical companies and CDMOs due to compliance requirements, capacity optimization strategies and demand for development and manufacturing services.
- The dominance of the United States can be attributed to factors such as high firm concentration of biotech companies, improving biologics pipelines, and robust investment in next-generation therapies, prompting pharmaceutical companies to access specialized CDMOs that provide critical advantages in product scaling, speed to commercialization, and flexible operations.
- Asia Pacific is registering rapid growth fueled by competitive manufacturing costs, developing pharmaceutical manufacturing capabilities, and buoyant government initiatives, helping the region attract global outsourcing contracts as well as build upon existing capabilities for drug substance manufacturing, biologics manufacturing and large-scale commercial manufacturing operations.
- Manufacturing services continue to be the leading service segment as pharmaceutical companies increasingly outsource large-scale production to deal with capacity constraints, reduce operation expenditure, and ensure regulatory compliance, and CDMOs invest in automation, continuous manufacturing technology and flexible production systems.
- Large molecular compounds and biologics have remained at the forefront amid the rise of various approvals of targeted therapy agents and monoclonal antibodies, with pharmaceutical and biotechnology companies requiring specialized infrastructure, technical know-how, and manufacturing scale-up for the development of complex biologic drug development processes through CDMOs.
- There is robust growth for injectable dosage forms, driven by the expanding demand for biologics, vaccines, and other high-end products requiring sterile filling environments, which motivates CDMOs to invest heavily in advanced containment technologies to meet the requirements for sterile products.
- Pharmaceutical companies will continue to remain the major end users as they incorporate more and more outsourcing strategies to concentrate more on research activities, minimize capital expenditures, and enhance speed-to-market, while long-term relationships will endure to bolster supply chain security and commercialization of complex drug products.
So, The Pharmaceutical Contract Development and Manufacturing Organization Market refers to outsourced services supporting the drug development, manufacturing, testing, and packaging activities of active pharmaceutical ingredients and finished dosage forms. CDMOs provide specialized infrastructure and the technical expertise that support efficient drug commercialization and a reduced capital investment requirement for sponsors. This higher level of drug complexity and regulatory associated requirements drives a continuous need for integrated outsourcing solutions. The market is driven by increasing biologics development, rising clinical trial activity, and growing demand for flexible capacity. Asset-light operational models are increasingly put into use in pharmaceutical companies, where the scalable production and advanced technologies necessary for such products are outsourced from CDMOs. Thus, this shift in strategy enables companies to enhance their operational efficiencies while managing fluctuating product demand and development risks. Advances in process development, automation, and analytics further facilitate the capabilities of CDMOs across all development stages. The rise of cell and gene therapies, biosimilars, and ultrapotent APIs further compels specialized high-containment manufacturing environments. This diversification, within pharmaceutical pipelines, enables CDMOs to be seen as strategic partners and not just service providers for the global pharmaceutical ecosystem.
Pharmaceutical Contract Development and Manufacturing Organization Market Segmentation
By Service Type
- Drug Development Services
Drug development services offer assistance to carry out early-stage research, formulation development, process optimization, and clinical trial material manufacturing. Outsourcing of drug development is increasing among pharmaceutical companies and biotech firms, which is fueling the market for drug development services, aiming to reduce product development time and R&D costs. Complexity of drugs like biologics and specialty drugs is further fuelling the market.
- Manufacturing Services
Manufacturing services form an essential part of the revenue segment, including the bulk manufacturing of APIs and dosage forms. Pharmaceutical companies are adopting outsourcing as a means to increase efficiency in operations and address challenges related to capacity. This segment is further driven by the increasing demand for flexible manufacturing infrastructure and compliance with major regulatory standards.
- Fill & Finish Services
Fill and finish services, on one hand, entail sterile filling, packaging, and preparation of drug formulations, especially injectables and biologics. An increase in the pipeline of biological and vaccine development projects has created a high requirement for specialized aseptic services. CDMOs with cutting-edge aseptic and automated technologies have emerged as leaders in these services.
- Analytical & Testing Services
These services provided by analytical and testing companies ensure safety, quality, and compliance of products. As regulatory requirements increase and formulations of drugs become complex, the demand for advanced analytical capabilities also grows. CDMOs who offer integrated testing services can aid pharmaceutical sponsors in hastening approval and development.
- Packaging Services
Packaging services cover all phases, including primary, secondary, labeling, and serialization of pharmaceutical products. This kind of growth is driven further by the increasing demand for patient-centric solutions in pharmaceutical product packaging. Outsourcing various pharmaceutical services, including pharmaceutical product packaging, helps these organizations improve their distribution efficiency
By Product Type
- Small Molecule
Small molecules still remain dominant in the CDMO market, with established process technologies and high demand. The manufacturing process of generic drugs, as well as strategies for lifecycle management, provide traction for the outsourcing industry. Cost efficiencies still remain a major driver for CDMOs.
- Large Molecules
Large molecules exhibit a growing trend, especially with the increase in biologic approvals and targeted therapies. Complexity associated with the manufacturing of large molecules, as well as the high investment demands, accelerate the adoption of specialized capabilities within the CDMO space for pharmaceutical firms. This is particularly prominent for monoclonal antibodies and other advanced biologic therapies.
- Highly Potent APIs
HPAPIs require specialized dealing offices, which decelerate the in-house manufacturing of these compounds for several organizations. A rise in the development of oncology medicine is one of the major growth drivers for this market. CDMOs with superior technology for dealing with HPAPIs have seen increasing demand.
- Biosimilars
Biosimilars segment growth is fueled by patent expirations of major biologics and cost pressure in healthcare systems. A major share for CDMOs exists in process development and optimization of large-scale manufacturing. Increasing adoption rate in emerging markets is another factor driving the segment
To learn more about this report, Download Free Sample Report
By Dosage Form
- Solid dosage forms
Solid dosage forms continue to be popular due to patient convenience, stability, and cost-effective manufacturing. Increasing demand for tablets and capsules in chronic disease management products will continue to drive stable outsourcing. CDMOs with capabilities for large-scale production and formulation will remain well-positioned in this segment.
- Injectable Dosage Forms
Injectables are seeing significant growth driven by the development of biologics, vaccines, and specialty therapeutics. “The need for sterile manufacturing and the current level of regulatory complexity will only continue to drive the trend of outsourcing to CDMOs to partner with companies that specialize in complex manufacturing. Continued investment in aseptic process development and high-speed filling
- Oral Liquids
Oral liquids are popular choices in the pediatric and elderly patient populations, thus supporting a consistent demand curve. The CDMOs offer flexibility in formulation and taste masking technology, enhancing patient compliance. The growth is driven by strong demand for customized product formulations.
- Topical Formulations
Topical formulations comprise creams, gels, and ointments applicable in dermatology and pain management-related segments. This area is affected by mounting health awareness among consumers and over-the-counter products. CDMOs help these products via their technological capabilities.
- Inhalation Formulations
Inhalation formulations require specific drug delivery systems and manufacturing capabilities. Increasing incidences of respiratory-related disorders and inhalation therapy adoption create a high demand for outsourcing. CDMOs offering device integration capabilities are also gaining prominence in the segment.
By End User
- Pharmaceutical Companies
The pharmaceutical industries form the single largest end-user community owing to the preponderance of outsourcing activities in the domain. CDMOs are increasingly being employed to lower business expenses and speed up productization. Strategic partnerships are common inthis space to enable business growth and meet internationalized supplies.
- Biotechnology Companies
Biotech businesses heavily depend on CDMOs as they lack manufacturing infrastructure. The rising innovation in biologics and cell therapies increases the demand for further biotech product development and production services. CDMOs allow biotech industries to scale up without demanding significant investment.
- Generic Drug Manufacturers
For generic drug manufacturers, CDMOs are important for optimizing cost of production and time to market. Large numbers of competitors in generic drugs drive companies to outsource services, mainly for manufacturing and analysis. This business is highly competitive, and generic drug manufacturers face downward price pressure, which is driving the need for outsourcing services.
- Virtual Pharma Companies
Virtual pharma companies have low internal infrastructural requirements and rely heavily on their outsourcing partners. CDMOs offer full-services from development to commercialization, hence facilitating asset-light companies. This segment is fueling growth as venture-backed pharmaceutical companies increase.
Regional Insights
North America accounts for a considerable market share due to advanced pharmaceutical research infrastructures and strong outsourcing adoption. The United States is the primary focus of regional activity, with high levels of biologics development and a strong presence of major pharmaceutical companies. Supportive manufacturing and supply chain expansion take place in Canada and Mexico. Europe, which includes major markets such as Germany, the United Kingdom, France, Spain, Italy, and the rest of Europe, is a mature market that is well supported by established pharmaceutical manufacturing capabilities and regulatory harmonization. Major innovation hubs are provided by Germany and the UK, while other European countries contribute biosimilar development and specialized manufacturing competencies. Asia-Pacific, which comprises Japan, China, Australia & New Zealand, South Korea, India, and the rest of Asia-Pacific, has shown rapid expansion in terms of increased cost competitiveness and the domestic industry demand for pharmaceuticals, as well as favorable government policies. China and India lead in API and generic production, whereas Japan and Korea lead in advanced biologics production and innovation-based outsourcing demand.
South America, with countries like Brazil and Argentina, is experiencing moderate growth with an increase in pharmaceutical product demand. New initiatives for local manufacturing are boosting growth. The Middle East & Africa region, including countries like Saudi Arabia, United Arab Emirates, South Africa, and the rest of the Middle East & Africa, is experiencing gradual growth with an increase in healthcare infrastructure initiatives and local manufacturing capabilities.
To learn more about this report, Download Free Sample Report
Recent Development News
- December 2024, Fraunhofer UMSICHT announced the presentation of materials based on fungal mycelium at the upcoming BAU 2025 trade fair. Therefore, there is clear interest in establishing the application of biogenic materials for their sustainability. This is the start of interest in the application of bio-based materials for the enhancement of sustainability, productivity, and building development.
- In April 2024, Biomason has announced the selection of Camilo Restrepo as its new Chief Executive Officer to support the further commercialization of its biotech-based cement alternatives. The change in leadership at the company signifies the firm’s efforts to achieve larger-scale industrial adoption of its low-carbon, biotech-based cement products. The recent development has marked another area of investment for the company to further develop its living building materials for the wider construction industry.
|
Report Metrics |
Details |
|
Market size value in 2025 |
USD 198.00 Billion |
|
Market size value in 2026 |
USD 210.00 Billion |
|
Revenue forecast in 2033 |
USD 360.00 Billion |
|
Growth rate |
CAGR of 8.00% from 2026 to 2033 |
|
Base year |
2025 |
|
Historical data |
2021 – 2024 |
|
Forecast period |
2026 – 2033 |
|
Report coverage |
Revenue forecast, competitive landscape, growth factors, and trends |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
United States; Canada; Mexico; United Kingdom; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; United Arab Emirates |
|
Key company profiled |
Lonza Group Ltd, Thermo Fisher Scientific Inc, Catalent Inc, WuXi AppTec Co Ltd, WuXi Biologics, Samsung Biologics Co Ltd, Recipharm AB, Siegfried Holding AG, Almac Group Ltd, Aenova Holding GmbH, FAMAR Health Care Services, Cambrex Corporation, FUJIFILM Diosynth Biotechnologies U.S.A., Inc, AGC Biologics and Pfizer Inc |
|
Customization scope |
Free report customization (country, regional & segment scope). Avail customized purchase options to meet your exact research needs. |
|
Report Segmentation |
By Service Type (Drug Development Services, Manufacturing Services, Fill & Finish Services, Analytical & Testing Services, Packaging Services), By Product Type (Small Molecules, Large Molecules, Highly Potent APIs, Biosimilars), By Dosage Form (Solid Dosage Forms, Injectable Dosage Forms, Oral Liquids, Topical Formulations, Inhalation Formulations) and By End User (Pharmaceutical Companies, Biotechnology Companies, Generic Drug Manufacturers, Virtual Pharma Companies) |
Key Pharmaceutical Contract Development and Manufacturing Organization Company Insights
Lonza Group Ltd represents a market leader in the Pharmaceutical CDMO market, differentiated by an extensive range of biologics, cell, gene therapy, and small molecules manufacturing capabilities. The company offers a broad range of differentiated services, from early-stage development through late-stage commercial manufacture, addressing complex therapeutic modalities. The investments of the company in an integrated platform have resulted in driving robust client engagement with pharmaceutical partners around the world. The geographical presence of the company is extensive, with facilities located across major markets. The company's recent performance reflects robust revenue growth, demonstrating market leadership from macro headwinds.
Key Pharmaceutical Contract Development and Manufacturing Organization Companies:
- Lonza Group Ltd
- Thermo Fisher Scientific Inc
- Catalent Inc
- WuXi AppTec Co Ltd
- WuXi Biologics
- Samsung Biologics Co Ltd
- Recipharm AB
- Siegfried Holding AG
- Almac Group Ltd
- Aenova Holding GmbH
- FAMAR Health Care Services
- Cambrex Corporation
- FUJIFILM Diosynth Biotechnologies U.S.A., Inc
- AGC Biologics
- Pfizer Inc.
Global Pharmaceutical Contract Development and Manufacturing Organization Market Report Segmentation
By Service Type
- Drug Development Services
- Manufacturing Services
- Fill & Finish Services
- Analytical & Testing Services
- Packaging Services
By Product Type
- Small Molecules
- Large Molecules
- Highly Potent APIs
- Biosimilars
By Dosage Form
- Solid Dosage Forms
- Injectable Dosage Forms
- Oral Liquids
- Topical Formulations
- Inhalation Formulations
By End User
- Pharmaceutical Companies
- Biotechnology Companies
- Generic Drug Manufacturers
- Virtual Pharma Companies
Regional Outlook
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- Japan
- China
- Australia & New Zealand
- South Korea
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
- Rest of the Middle East & Africa










 APAC:+91 7666513636
APAC:+91 7666513636





