Market Summary
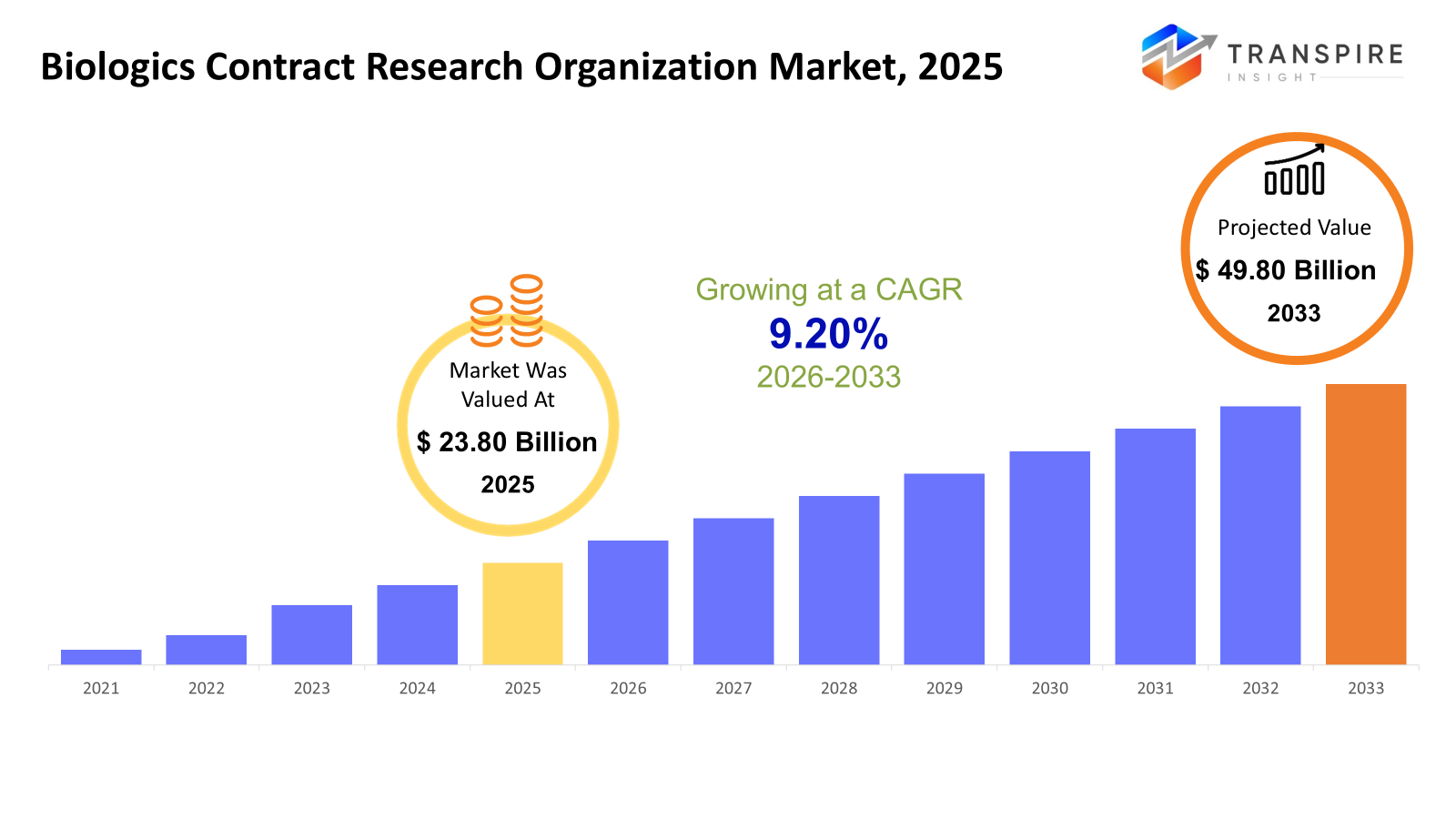
The global Biologics Contract Research Organizations market size was valued at USD 23.80 billion in 2025 and is projected to reach USD 49.80 billion by 2033, growing at a CAGR of 9.20% from 2026 to 2033. Growth in AI for factory automation spreads fast because more places adopt smart production methods along with the Industry 4.0 movement worldwide. Machines now think better thanks to artificial intelligence, helping spot breakdowns early, smooth out workflows, check product quality, improve delivery routes, cutting waste while lifting output. As robots link up with sensors and learning systems on shop floors, interest grows stronger every quarter.
Market Size & Forecast
- 2025 Market Size: USD 23.80 Billion
- 2033 Projected Market Size: USD 49.80 Billion
- CAGR (2026-2033): 9.20%
- North America: Largest Market in 2026
- Asia Pacific: Fastest Growing Market
To learn more about this report, Download Free Sample Report
Key Market Trends Analysis
- The North American market share is estimated to be approximately 46% in 2026. Home to cutting-edge biotech labs, North America holds a strong edge. Clinical studies unfold at a fast pace across its research hubs. Outsourcing plays a big role here, widely embraced by industry players.
- Fueled by cutting-edge biologics research, the United States leads worldwide contributions. Its robust network of contract research organizations helps power that position.
- Out here in Asia Pacific, growth does not slow; biotech keeps stretching wider. Lower expenses play a part, sure, yet it’s the push into new studies that really shifts things. Money flowing into clinical work adds fuel, but so does local momentum. Progress is not just happening, it’s building quietly, step by uneven step.
- Clinical Trial Services share approximately 76% in 2026. Right now, clinical trial services take the lead because more biopharmaceutical companies hand off intricate human research. Instead of handling everything in-house, they rely on outside experts for these demanding tasks.
- Starting strong in therapy zones, monoclonal antibodies lead because research pushes hard here. Their reach across treatments grows, fueled by steady science work instead of quick wins.
- Fueled by rapid advances in biologic drugs, biotech firms lead the pack. Their need to outsource tasks pushes them ahead, setting a clear pace. Growth here ties closely to fresh scientific strides. Pressure to stay competitive keeps momentum high.
Growth in the worldwide market for biologics contract research groups is picking up speed. This happens because making biologic drugs gets more complex every year. At the same time, there’s a stronger need to hire outside teams for studies and trial work. Drug makers and bio firms now turn more often to these external partners. They do so not just to save money but also to move faster through development phases. Expertise matters too - especially in fields like engineered antibodies or modified cells used in therapy. By working with specialists, companies can shift attention back to their main strengths. These third-party labs bring advanced tools plus wide-reaching connections across regions. Their support spreads from early lab exploration right into human testing stages. What results is a shared path forward without carrying all tasks internally.
Right now, clinical trials take up the biggest chunk of the market, and they are expanding quickly - because companies making biological drugs turn to specialists who handle every stage of human testing, meet strict rules, and stay on track with deadlines. Before any drug reaches that phase, early research work fuels progress too; more labs run rapid screenings, analyze molecules, and dig into genetic and protein data. Then there are advisors focused solely on regulations - they help sponsors navigate thick layers of approvals. These experts make outsourcing smarter, stitch trust deeper into collaborations.
Biotech firms lead the way, their need for quick development pushing demand higher because of new advances in biological drugs. Big drug makers follow close behind, relying on outside help during initial project stages more often now. Universities and science centers add momentum too, using third-party labs to handle complex tests and rules-related work. Government-backed facilities do much the same, turning to expert teams when precision matters most. Monoclonal antibodies draw attention, helped by rising worldwide interest in advanced treatments. Vaccines keep pace, riding the wave of increased investment and faster approvals. Cell and gene therapies grow steadily, opening fresh paths where older methods once fell short.
North America sits at the front, thanks to strong biopharmaceutical systems, well-built clinical testing setups, backed by heavy spending on innovation, most of it coming from the United States. Not far behind, Europe keeps moving forward because rules push companies to stay aligned, helped along by long-standing science centers and university-driven studies. Speeding up fast, the Asia Pacific region grows quickest as its biotech fields widen, benefitting from lower operating costs, pulled ahead by more labs hiring outside help for biological trials and development work. Meanwhile, places like Latin America, plus parts across the Middle East and Africa, slowly step into view, where signs show more contract research groups getting hired each year.
Biologics Contract Research Organizations Market Segmentation
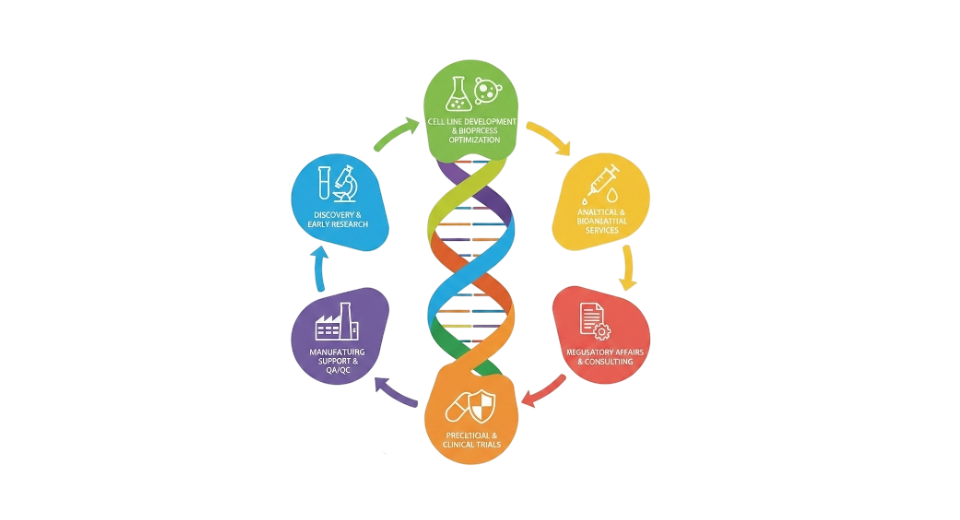
By Service Type
- Discovery Services
Help comes early through research guidance, finding targets, and shaping leads. One step at a time, progress builds where ideas meet testing. Focus shifts often, yet direction holds steady. What matters appears slowly, revealed by trial after quiet trial.
- Preclinical Services
Beyond early lab work, scientists run tests on living systems or cells to check how safe and effective a treatment might be. These steps happen long before people are involved in studies. Some evaluations take place inside organisms, others outside in controlled environments. Each method helps spot risks or benefits ahead of human testing.
- Clinical Trial Services
Running tests on people to see how well biological medicines work. Overseeing every step of those studies closely. Making sure rules are followed during each trial phase. Watching details from start to finish without missing anything. Keeping data accurate through careful tracking methods. Moving forward only when safety checks pass completely.
- Regulatory Support & Consulting
From handling paperwork to meeting rules, help comes step by step. One part deals with filings, another checks alignment. Guidance shapes the path forward, piece by piece.
- Lab Services
Testing happens here using biology, genes, or proteins - part of building advanced medicines. Work includes measuring biological responses alongside genetic patterns while tracking protein behavior during development.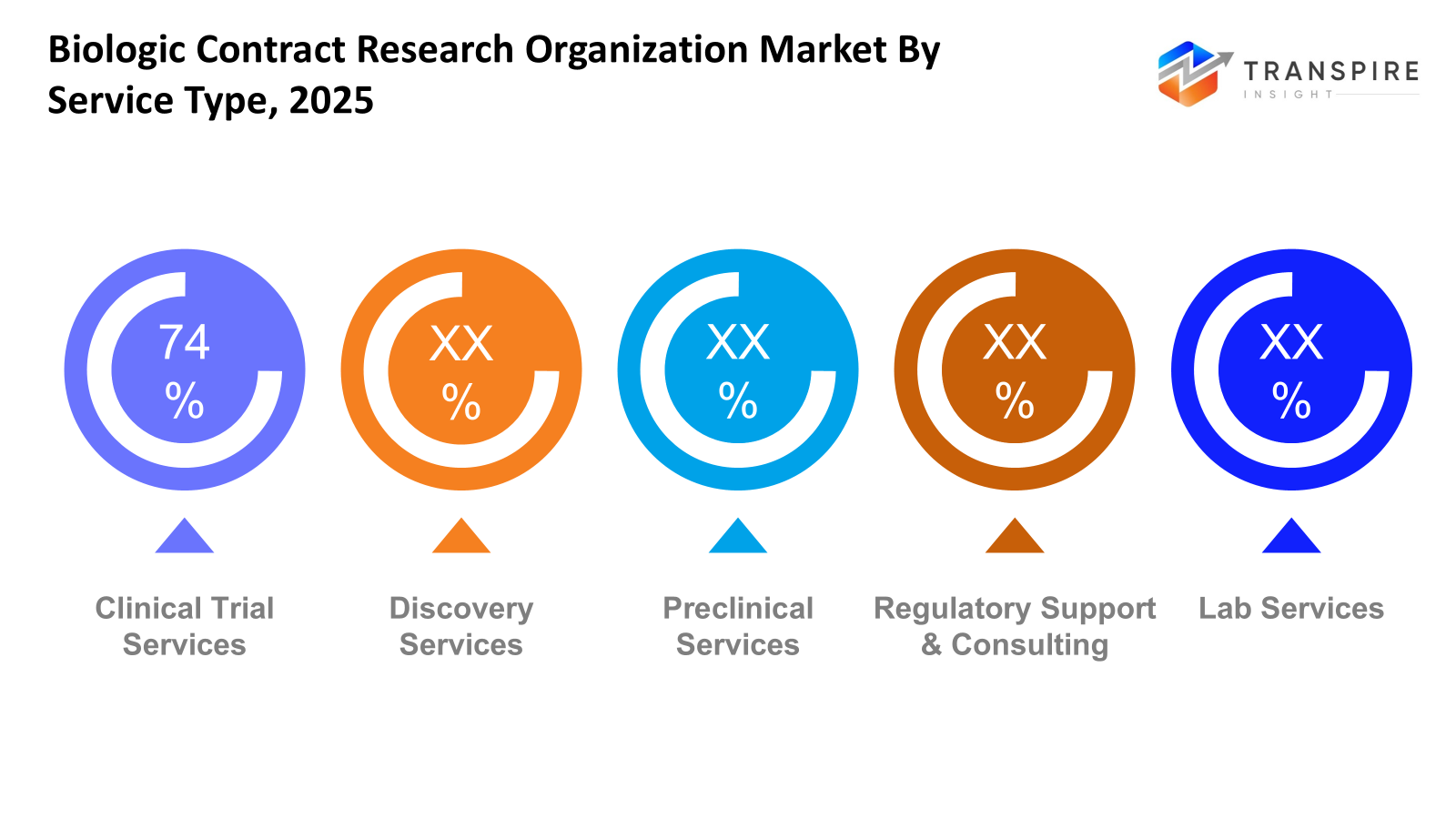
To learn more about this report, Download Free Sample Report
By Therapeutic Area
- Monoclonal Antibodies
CRO support for the development and testing of monoclonal antibody therapies.
- Recombinant Protein
Lab work on engineered proteins includes checking how they behave, preparing them properly, and then running tests to confirm performance. Each step happens under controlled settings, so results stay reliable throughout the process.
- Cell & Gene Therapies
Therapy advances begin here, and tailored help for complex cell and gene projects unfolds quietly behind the scenes. Progress shows up in careful steps, not bold claims, guided by focused expertise. New paths emerge through steady work, backed by deep knowledge of intricate processes. Results grow from attention to detail, supported by experience that others skip. Breakthroughs wait patiently; preparation makes them possible.
- Vaccines
Work on new options begins with digging through data, then checking results in controlled settings. Trials follow each step, shaped by careful observation, one phase built on what came before it. Support flows behind every stage, steady but unseen.
- Others
There’s support for those. Newer biological treatments also fall under available offerings. Help exists across these specialized areas.
By End-Users
- Pharmaceutical Companies
Outsource biologics research and clinical programs to CROs.
- Biotechnology Companies
Some biotech firms lean on contract research groups to push new biological treatments forward through testing phases. One after another, these partnerships tackle complex lab work while keeping pace with tight development timelines.
- Academic & Research Institutes
Use CRO services for complex biologics research projects.
- Government & Contract Research Laboratories
Public labs often turn to outside groups when big trials are needed. Not just size - rules matter too. So they bring in specialists who know the process. These teams handle work that must meet strict standards. Scale pushes them outward. Rules push them toward experts. Outsiders step in where both volume and oversight grow.
Regional Insights
Fueled by dense clusters of drugmakers and biopharmaceutical firms, North America leads the global Biologics CRO landscape. A mature network for running clinical trials gives the region an edge, alongside heavy spending on research. Within it, the United States stands out as its swift shift toward externalized services plays a big role. Clear rules set by authorities help too. So does its deep commitment to pushing forward treatments like engineered antibodies, immunizations, and genetic-level remedies.
Across Europe, the market remains large, thanks to well-established biotech centers, tough rules on safety, a solid history of university-led science, and deep medical trial expertise. Nations like Germany, the United Kingdom, and France stand out as drugmakers there, plus labs now rely more on outside groups to handle biological drug work and testing rounds, aiming for smoother operations while meeting standards.
Growth speeds ahead fastest across Asia Pacific, thanks to a surge in biotech plus pharma work, lower costs popping up everywhere, along with more clinical studies getting underway. China, India, and Japan are strongly backed by state-driven programs, deeper spending on research efforts, and a swelling number of trained experts joining the field. Over in Latin America and parts of the Middle East and Africa, new opportunities unfold slowly as interest builds for help running biological drug studies, managing trials, and meeting complex rules.
To learn more about this report, Download Free Sample Report
Recent Development News
- January 16, 2026 – WuXi Biologics launched the PatroLab digital twin platform to transform biologics manufacturing.
- June 16, 2025 – Samsung Biologics launched Samsung Organoids to expand its portfolio beyond CDMO.
(Source: https://www.koreabiomed.com/news/articleView.html?idxno=27899)
|
Report Metrics |
Details |
|
Market size value in 2025 |
USD 23.80 Billion |
|
Market size value in 2026 |
USD 27.00 Billion |
|
Revenue forecast in 2033 |
USD 49.80 Billion |
|
Growth rate |
CAGR of 9.20% from 2026 to 2033 |
|
Base year |
2025 |
|
Historical data |
2021 – 2024 |
|
Forecast period |
2026 – 2033 |
|
Report coverage |
Revenue forecast, competitive landscape, growth factors, and trends |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
United States; Canada; Mexico; United Kingdom; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; United Arab Emirates |
|
Key company profiled |
Lonza Group AG, WuXi Biologics (WuXi AppTec), Samsung Biologics, Catalent, Inc., Fujifilm Diosynth Biotechnologies, AGC Biologics, Thermo Fisher Scientific (Patheon), Boehringer Ingelheim BioXcellence, Charles River Laboratories, Parexel International, IQVIA Holdings Inc., Medpace Holdings, Syngene International, Eurofins Scientific, KBI Biopharma, LabCorp Drug Development, and ICON plc. |
|
Customization scope |
Free report customization (country, regional & segment scope). Avail customized purchase options to meet your exact research needs. |
|
Report Segmentation |
By Service Type (Discovery Services, Preclinical Services, Clinical Trial Services, Regulatory Support & Consulting, Lab Services), By Therapeutic Area (Monoclonal Antibodies, Recombinant Protein, Cell & Gene Therapies, Vaccines, Others), By End-Users (Pharmaceutical Companies, Biotechnology Companies, Academic & Research Institutes, Government & Contract Research Laboratories) |
Key Biologics Contract Research Organizations Company Insights
One name stands out when talking about making biological medicines at scale: Lonza Group AG. Not just any lab, this firm builds treatments using living cells and genes, along with targeted proteins and protective shots against disease. From early idea to testing in humans, they walk beside drug developers every step. Their labs stretch across continents, equipped to handle strict rules while pushing what's possible. Speed matters, so they shape processes that cut waiting periods without cutting corners. Big pharma leans on them; small startups trust them, too. Growth is not accidental; they plan new spaces, welcome fresh ideas, and team up where it counts. Watch closely, and you’ll see how deeply they are woven into modern medicine’s fabric. Few match their mix of skill, spread, and steady progress.
Key Biologics Contract Research Organizations Companies:
- Lonza Group AG
- WuXi Biologics (WuXi AppTec)
- Samsung Biologics
- Catalent, Inc.
- Fujifilm Diosynth Biotechnologies
- AGC Biologics
- Thermo Fisher Scientific (Patheon)
- Boehringer Ingelheim BioXcellence
- Charles River Laboratories
- Parexel International
- IQVIA Holdings Inc.
- Medpace Holdings
- Syngene International
- Eurofins Scientific
- KBI Biopharma
- LabCorp Drug Development
- ICON plc.
Global Biologics Contract Research Organizations Market Report Segmentation
By Service Type
- Discovery Services
- Preclinical Services
- Clinical Trial Services
- Regulatory Support & Consulting
- Lab Services
By Therapeutic Area
- Monoclonal Antibodies
- Recombinant Protein
- Cell & Gene Therapies
- Vaccines
- Others
By End-Users
- Pharmaceutical Companies
- Biotechnology Companies
- Academic & Research Institutes
- Government & Contract Research Laboratories
Regional Outlook
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- Japan
- China
- Australia & New Zealand
- South Korea
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
- Rest of the Middle East & Africa









 APAC:+91 7666513636
APAC:+91 7666513636





