Market Summary
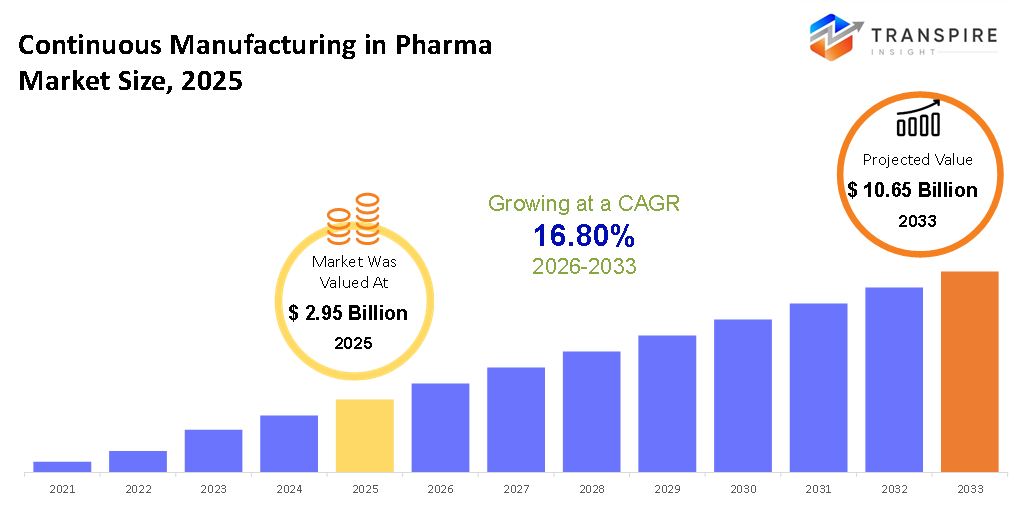
The global Continuous Manufacturing in Pharma market size was valued at USD 2.95 billion in 2025 and is projected to reach USD 10.65 billion by 2033, growing at a CAGR of 16.80% from 2026 to 2033. The market is growing at a high CAGR owing to rising need for effective and scalable superior quality pharmaceutical production. Regulatory agencies in North America and Europe are increasingly promoting continuous pharmaceutical manufacturing to enhance safety and reduce failure rates. Use of state-of-the-art technology such as flow reactors and PAT leads to higher productivity and product quality. Additionally, rise in the pharmaceutical manufacturing in Asia Pacific and emerging nations is driving the market.
Market Size & Forecast
- 2025 Market Size: USD 2.95 billion
- 2033 Projected Market Size: USD 10.65 billion
- CAGR (2026-2033): 16.80%
- North America: Largest Market in 2026
- Asia Pacific: Fastest Growing Market
To learn more about this report, Download Free Sample Report
Key Market Trends Analysis
- North America is really taking to this idea of manufacturing. The main reason is that the government is giving companies incentives to do. Also North America has an infrastructure which helps a lot. This means that pharmaceutical companies can make their products more efficiently which saves them money. At the time they can make sure that the quality of their products like the actual medicine and the final packaged products is always the same. This is true, for both the ingredients that go into the medicine and the finished products that people buy. North America and continuous manufacturing are a pair because of this.
- In the U.S. people are starting to use flow reactors a lot in pharmaceutical plants. This is helping them make molecule drugs faster. They can also control the reaction parameters precisely. It is easier to make a lot of these high demand therapeutic products with continuous flow reactors. Companies can make more of the flow reactors to meet the high demand for these products, which is a big advantage of using continuous flow reactors in pharmaceutical plants.
- The U.S. is seeing more companies use process technology. This technology helps the U.S. monitor the quality of things in time. It looks at the things that can affect the quality of a product. The U.S. is using this technology to reduce mistakes when making batches of products. It is also helping the U.S. follow the rules set by the Food and Drug Administration. The Food and Drug Administration has rules, for making all kinds of products including molecules and biologics. The U.S. is using process technology for both types of production.
- In Asia Pacific countries like India and China people are putting money into making continuous manufacturing facilities, for Active Pharmaceutical Ingredients and finished medicines. The government is helping with this. People really want to buy good quality medicines that are not too expensive both in their own countries and when they are sold to other countries.
- The Asia Pacific region is seeing a lot of companies use flow reactors when they make chemicals. This is a thing because it helps the reactions work better uses less energy and makes it easier to make more of the drugs that people need. The Asia Pacific region is using flow reactors to make small molecule drugs faster. This is important because more people, in the Asia Pacific region need these drugs to get better.
- It also reflects a particular tendency towards Process Analytical Technologies for making biologics and vaccines, and this helps achieve quality monitoring and enables quicker launch times for emerging regions for pharmaceuticals.
Global continuous manufacturing in pharma is a way of making medicines. This is where the main ingredients and the final products are made in a process. They are made one after the other in a workflow. This is different from the way of making medicines in batches.
Continuous manufacturing is a way because it helps make medicines faster and cheaper. The quality of the medicines is also better. Pharmaceutical companies are starting to use manufacturing because people need good medicines and they need them fast. Companies want to get their medicines to the market. That is why continuous manufacturing in pharma is becoming very popular with companies all over the world.
The way we make things change because of technology. We are using something called flow reactors and process analytical technology. These things help us make chemicals in a precise way. This means we can make more of what we want. It is better quality. We can also watch what is happening as we make it so we can stop things going wrong. We use these methods to make all sorts of drugs like the ones you take as a pill and the ones that are made from living things, like vaccines. This shows that we can use manufacturing to make lots of different types of drugs. Big drug companies are putting money into facilities and systems that do things in a mix of old and new ways. They want to make sure they meet the strict rules that are in place. At the time they need to make more medicine because a lot of people need it. The companies are using a combination of batch and continuous processes to get this done. They are doing this so they can keep up with the growing demand from patients and follow the rules that are set by the regulators. Big pharmaceutical companies are constantly looking for ways to upgrade and make things better.
From a geographic standpoint, the current market leaders are North America and Europe, which have better regulatory support, even more advanced infrastructure, and earlier adopters of continuous manufacturing technology. As the trend unfolds, the rapidly growing geographic region is the Asia-Pacific region, which has already started to make significant investments in India, China, and Japan. South America and the Middle East/Africa regions are experiencing a gradual adoption process.
Continuous Manufacturing in Pharma Market Segmentation
By Product
- APIs (Active Pharmaceutical Ingredients)
APIs are dominating the continuous manufacturing process because of the requirement for high-quality, scalable production with reduced batch failures.
- Finished Dosage Forms (FDFs)
While finished dosage forms adoption is growing, as companies pursue consistent quality, faster throughput and cost-efficient tablet, capsule and liquid production.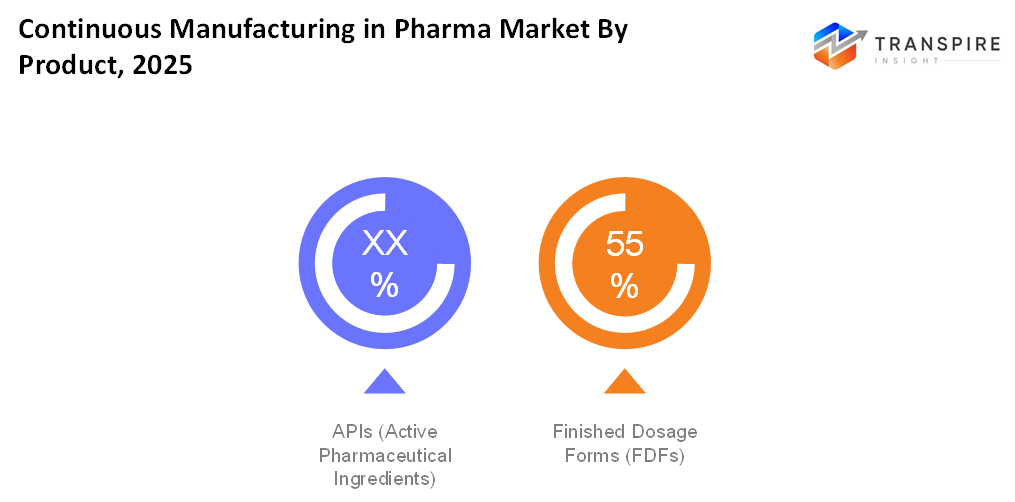
To learn more about this report, Download Free Sample Report
By Technology
- Continuous Flow Reactors
Continuous flow reactors are widely adopted for the purpose of achieving high precision in chemical syntheses, process efficiency, and scalability.
- Process Analytical Technology (PAT)
Process analytical technology can assure critical quality attribute monitoring in real time, decrease batch errors, and ensure regulatory compliance; hence, both are becoming indispensable in modern pharmaceutical production.
By Drug Type
- Small Molecule Drugs
Small molecules continue to be the leading segment, driven by their relatively simple chemistry, strong demand, and adaptability to continuous processing.
- Biologics & Vaccines
biologics and vaccines are undergoing rapid evolution, based on demands such as reproducibility, rapid production, and scalability regarding complex therapeutics.
Regional Insights
The global continuous manufacturing in pharma market is different in parts of the world. This difference is because of things like how strict the rulers what the infrastructure is like and how much medicine is being made in each place. North America is the leader when it comes to manufacturing in pharma and the U.S. is the biggest player. This is because the U.S. has rules that support this kind of manufacturing it has infrastructure and big pharmaceutical companies started using this method early on. Canada and Mexico are also important. They are not as big as the U.S.. They benefit from working with the U.S. and from slowly starting to use new technology. Continuous manufacturing in pharma is growing in these countries. The continuous manufacturing in pharma market is doing well in North America in the U.S. because of its strong support, for this kind of manufacturing.
In Europe countries like Germany, the United Kingdom, France, Spain and Italy are really good at using things because they have very high standards for quality. They also like to try ideas and have good policies to help them do that. They are investing a lot of money in factories and equipment.
The rest of Europe is a bit different. These are countries like the ones I mentioned earlier. They are not as good at using new things. They are getting better. It is happening slowly. This is because they must follow a lot of rules and they are also thinking about how much things cost. The United Kingdom, France, Spain and Italy are all part of Europe. The rest of Europe is moving at a slower pace.
The Asia Pacific region is growing fast. This is because Japan, China, South Korea, India and Australia and New Zealand have a lot of pharmaceutical factories and are spending more money on new technologies. The Asia Pacific region has two parts. Japan, China, South Korea, India and Australia and New Zealand are the countries. The rest of the Asia Pacific region is also growing, Not as fast. This is because people in these countries are buying things and companies are making things to sell to other countries.
In South America, Brazil serves as the primary tier-1 market, while Argentina and the Rest of South America remain tier-2 with gradual modernization trends. The Middle East & Africa region is emerging, led by tier-1 markets such as Saudi Arabia, the UAE, and South Africa, while the rest of the region shows early-stage adoption driven by healthcare infrastructure development.
To learn more about this report, Download Free Sample Report
Recent Development News
- September 2025, Eli Lilly announced plans to build a major manufacturing facility in Goochland County, Virginia, with $5 billion of investment to expand domestic pharmaceutical production capacity, particularly for active pharmaceutical ingredients and advanced therapies. This reflects continued industry emphasis on bolstering manufacturing resilience and modern production capabilities within the U.S..
- April 2025, Swiss drugmaker Novartis revealed a $23 billion plan to expand its manufacturing footprint and R&D efforts in the U.S., including multiple new and expanded facilities. The move underscores growing industry focused on modernizing production and strengthening supply chains amid changing global trade dynamics.
(Source: https://www.ft.com/content/a46eff5f-7cf5-4a34-8e82-bc13ce3223cc)
|
Report Metrics |
Details |
|
Market size value in 2025 |
USD 2.95 billion |
|
Market size value in 2026 |
USD 3.60 Billion |
|
Revenue forecast in 2033 |
USD 10.65 billion |
|
Growth rate |
CAGR of 16.80% from 2026 to 2033 |
|
Base year |
2025 |
|
Historical data |
2021 – 2024 |
|
Forecast period |
2026 – 2033 |
|
Report coverage |
Revenue forecast, competitive landscape, growth factors, and trends |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; Mexico; United Kingdom; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; United Arab Emirates |
|
Key company profiled |
GEA Group, Thermo Fisher Scientific, Siemens AG, Glatt GmbH, L.B. Bohle Maschinen und Verfahren, Coperion GmbH, Korsch AG, Scott Equipment Company, Syntegon Technology, Chemtrix, Continuus Pharmaceuticals, Pfizer Inc., Novartis AG, Eli Lilly and Company, Merck & Co., Inc. |
|
Customization scope |
Free report customization (country, regional & segment scope). Avail customized purchase options to meet your exact research needs. |
|
Report Segmentation |
By Product (APIs, Finished Dosage Forms), By Technology (Continuous Flow Reactors, Process Analytical Technology), By Drug Type (Small Molecule Drugs, Biologics & Vaccines) |
Key Continuous Manufacturing in Pharma Company Insights
Thermo Fisher Scientific Inc. is a leading participant in the continuous manufacturing in pharma market because of its comprehensive offerings in the continuous manufacturing equipment, process analytical technology, and integrated digital solutions space. The firm enables API and finished dosage form manufacturing through leading-edge flow chemistry systems, real-time analytics, and automation platforms. Also, it is well focused on compliance with the regulatory bodies, and that aligns with global regulatory encouragement for continuous manufacturing adoption. Finally, Thermo Fisher's global presence, strong R&D investments, and partnerships with pharmaceutical manufacturers and CDMOs make it one of the critical enablers of scalable, efficient, and high-quality continuous pharmaceutical production.
Key Continuous Manufacturing in Pharma Companies:
- GEA Group
- Thermo Fisher Scientific
- Siemens AG
- Glatt GmbH
- B. Bohle Maschinen und Verfahren
- Coperion GmbH
- Korsch AG
- Scott Equipment Company
- Bosch Packaging Technology (Syntegon)
- Chemtrix
- Continuus Pharmaceuticals
- Pfizer
- Novartis
- Eli Lilly and Company
- Merck & Co., Inc.
Global Continuous Manufacturing in Pharma Market Report Segmentation
By Product
- APIs (Active Pharmaceutical Ingredients)
- Finished Dosage Forms (FDFs)
By Technology
- Continuous Flow Reactors
- Process Analytical Technology (PAT)
By Drug Type
- Small Molecule Drugs
- Biologics & Vaccines
Regional Outlook
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- Japan
- China
- Australia & New Zealand
- South Korea
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
- Rest of the Middle East & Africa










 APAC:+91 7666513636
APAC:+91 7666513636





