Market Summary
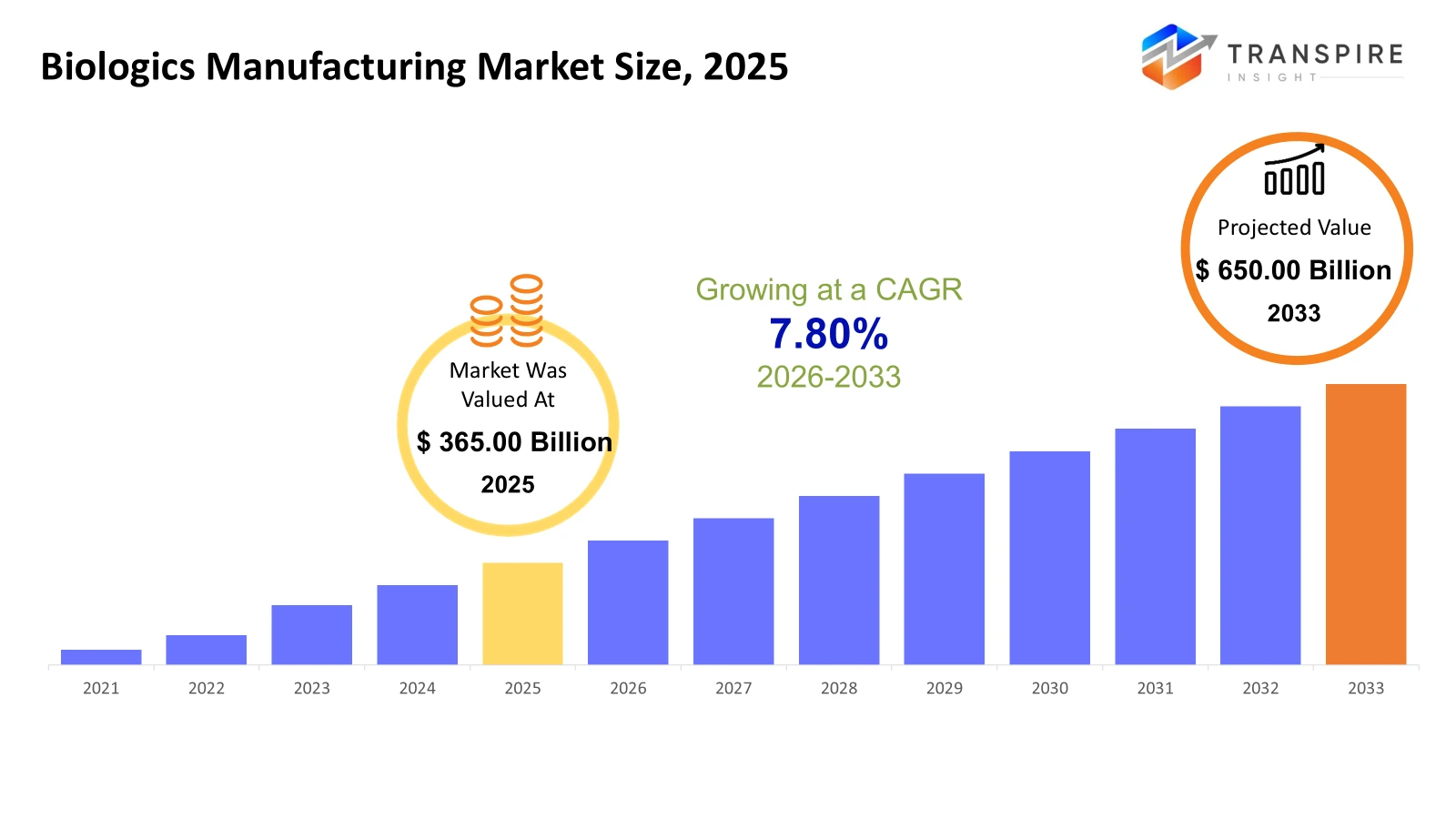
The global Biologics Manufacturing market size was valued at USD 365.00 billion in 2025 and is projected to reach USD 650.00 billion by 2033, growing at a CAGR of 7.80% from 2026 to 2033. The market for biologics manufacturing is projected to register a strong growth rate owing to the rising incidence of chronic and rare diseases, the growing use of monoclonal antibodies, and the rapid production of vaccines based on global health initiatives. Advances in cell culture, gene, and cell therapy technologies are also increasing the efficiency of manufacturing processes and decreasing costs, thus enabling increased production. Collaborations, contract manufacturing organization outsourcing, and the growing emerging market base also add to the scalability and adoption of the market. Furthermore, favorable regulatory policies and increased healthcare spending in North America and the Asia Pacific region are also facilitating fast-track approvals, thus contributing to the overall CAGR growth.
Market Size & Forecast
- 2025 Market Size: USD 365.00 Billion
- 2033 Projected Market Size: USD 650.00 Billion
- CAGR (2026-2033): 7.80%
- North America: Largest Market in 2026
- Asia Pacific: Fastest Growing Market
To learn more about this report, Download Free Sample Report
Key Market Trends Analysis
- North America is witnessing steady growth because of the well-developed R&D infrastructure, strong regulatory support, and high healthcare spending that is fueling the demand for biologics. Monoclonal antibodies account for the largest product demand, cell culture is the foremost technology, oncology is the largest application area, and CMOs are gaining popularity for scalable manufacturing.
- The United States is the major contributor to the dominance of the North American market, which is driven by oncology biologics, prevalent vaccine manufacturing, and recombinant protein use. Improved upstream and downstream technologies are enhancing process productivity, and CMOs and biotech companies are increasing capacity to cater to the growing therapeutic needs and regulatory requirements.
- The Asia-Pacific market is the fastest-growing market due to increasing healthcare spending, developing manufacturing facilities, and government support. Monoclonal antibodies, vaccines, and recombinant proteins are the major products, cell culture is the foremost technology, oncology is the largest application area, and contract manufacturing adoption is increasing rapidly for scalable biologics manufacturing.
- Monoclonal antibodies drive product adoption because of their strong efficacy in cancer and autoimmune diseases, with an increasing number of approvals for pipeline products and biosimilars that improve production demand in the North American market.
- Cell Culture Technology is the leading upstream processing technology, which offers high productivity and scalability for monoclonal antibody, recombinant protein, and vaccine production. Continuous innovation in bioreactor technology, development of optimal growth media, and single-use bioreactor technology are improving process efficiency and reducing costs.
- The Oncology market is the leading application area, driven by monoclonal antibodies, recombinant proteins, and emerging cell and gene therapies. The large number of cancer patients, an increasing pipeline of targeted therapies, and increased R&D investments are driving market growth.
- Contract Manufacturing Organizations (CMOs) are gaining popularity as a preferred choice for outsourcing biologics manufacturing. CMOs provide scalable manufacturing, regulatory knowledge, and cost savings, allowing pharmaceutical and biotech companies to accelerate product commercialization with reduced operational risk.
So, The biologics manufacturing market includes the production of complex therapeutic biologics such as monoclonal antibodies, recombinant proteins, vaccines, and advanced cell and gene therapies. These biologics are critical to the treatment of chronic, infectious, autoimmune, and rare diseases, which indicate the transition from traditional small-molecule therapies to high-value biologics. The biologics manufacturing market is driven by complex upstream and downstream processing techniques such as cell culture, microbial fermentation, chromatography, filtration, and purification to ensure product quality and regulatory requirements.
The growing incidence of diseases such as cancer, cardiovascular diseases, and rare genetic disorders has driven the need for targeted therapies. In addition, advancements in bioreactor technology, continuous manufacturing, and single-use bioreactors enhance operational efficiency and scalability. Contract manufacturing organizations enable pharmaceutical and biotechnology companies to lower production costs while ensuring regulatory compliance and faster time-to-market, which drives the growth of the market. Government initiatives, increased R&D spending, and the launch of biosimilars are further improving the market environment. New biologics such as cell and gene therapies are also finding favor in personalized medicine applications, thus opening new avenues for growth.
Biologics Manufacturing Market Segmentation
By Product Type
- Monoclonal Antibodies
Monoclonal antibodies lead the biologics market because of their high efficacy in targeted therapies, particularly in cancer and immunological diseases. The market is aided by increased R&D spending and the growing number of approvals for biosimilars worldwide.
- Recombinant Proteins
Recombinant proteins are essential for both therapeutic and diagnostic purposes, with significant adoption in the treatment of chronic diseases. Ongoing advancements in expression and purification methods improve productivity and lower production costs, thereby increasing market potential.
- Vaccines
Vaccines are of prime importance, particularly with the global focus on immunization initiatives. Advances in technology, such as mRNA and viral vector-based platforms, improve production productivity, while the growing need for infectious disease prevention fuels market growth.
- Cell Therapy Products
Cell therapy products are witnessing rapid growth due to their applications in regenerative medicine. Personalized medicine and advancements in cell culture technology improve clinical utility, although high costs and regulatory complexities hinder large-scale production.
- Gene Therapy Products
The gene therapy products market is a niche but high-value segment. The growth is fueled by the increasing number of clinical trials, the development of advanced vectors, and growing investment in the treatment of rare diseases, despite the manufacturing and regulatory hurdles.
- Others
Other biologics include peptides, oligonucleotides, and emerging biopharmaceuticals. These markets have moderate growth fueled by innovation in novel therapies and applications in areas outside the conventional treatment segments.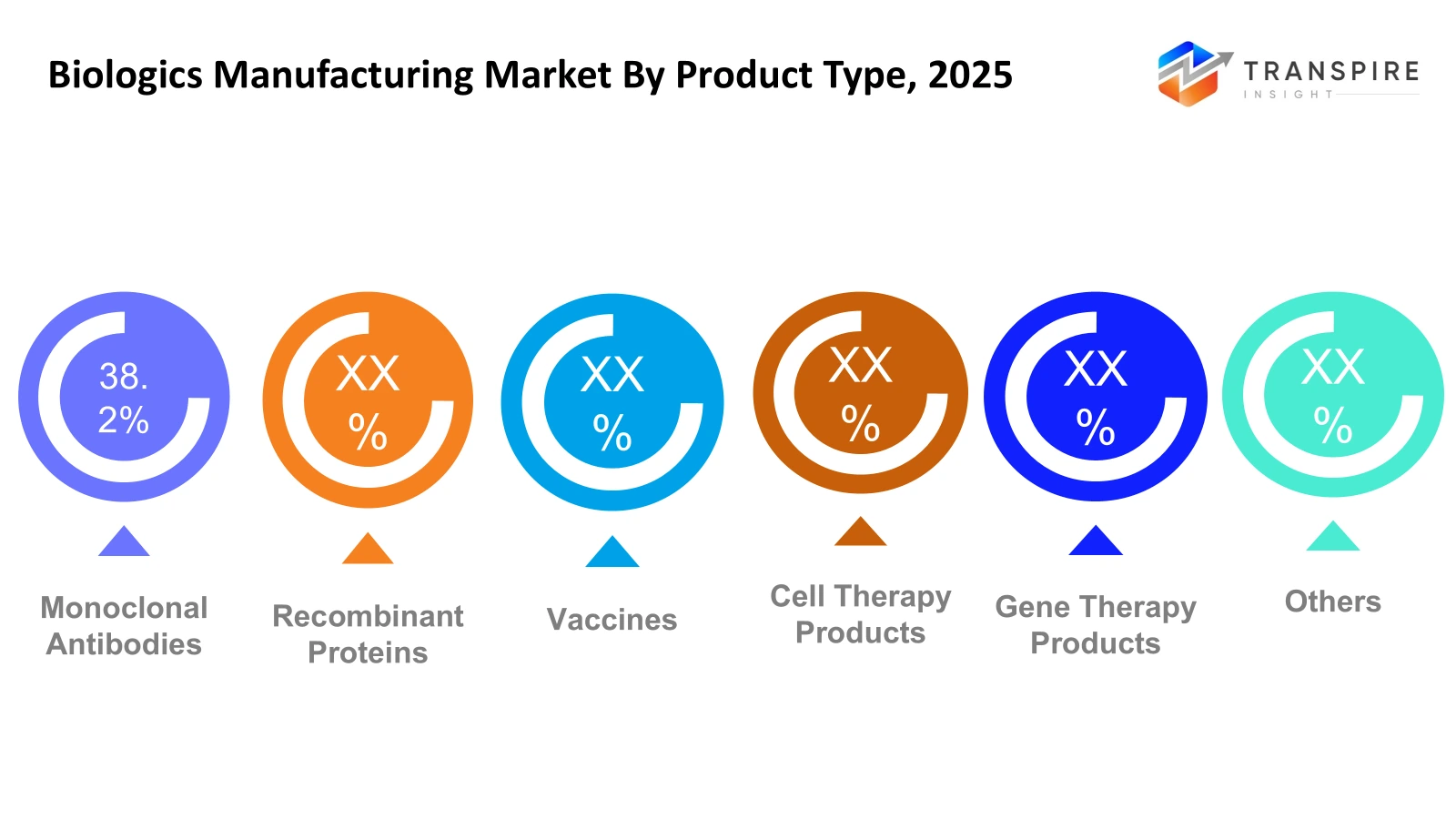
To learn more about this report, Download Free Sample Report
By Technology
- Cell Culture
Cell culture is the driving force in biologics manufacturing, offering scalable processes for protein and monoclonal antibody production. Advances in bioreactor technology and media development enhance yield and quality.
- Microbial Fermentation
Microbial fermentation is an economically viable method for the production of recombinant proteins and enzymes. Its advantages include high scalability and short growth cycles, especially for non-mammalian biologics.
- Chromatography
Chromatography is a key downstream processing technology for purification. Continuous and high-throughput processing enhances efficiency, ensuring product quality and regulatory requirements.
- Filtration
Filtration is beneficial for both upstream and downstream processes as it removes contaminants and ensures that the product is sterile. Improvements in membrane technology improve processing capacity and alleviate production bottlenecks.
- Purification
Purification technologies are used to ensure high-quality biologics. New resins and single-use technologies improve efficiency and minimize the risk of cross-contamination.
By Application
- Oncology
Oncology is a major driver for the adoption of biologics because of targeted therapies and immunotherapies. Rising incidence of cancer and investment in new monoclonal antibodies and CAR-T cell therapies make it the largest application area.
- Infectious Diseases
The demand for vaccines and antibody-based therapies for infectious diseases is strong. Breakouts, pandemics, and preventive vaccination programs are fueling the need for large-scale biologics manufacturing.
- Autoimmune Disorders
Biologics for autoimmune diseases such as rheumatoid arthritis and psoriasis are in widespread use because of their specificity and potency. The biologics pipeline and adoption of biosimilars are major growth drivers.
- Cardiovascular Diseases
Cardiovascular biologics, such as protein therapies, are experiencing rising demand owing to the growing number of cases related to cardiovascular diseases.
- Rare Diseases
Rare disease biologics are niche products with small patient bases. The growing number of orphan drug approvals and gene and cell therapy development is increasing this market despite the high cost of production.
- Others
Other areas of application include metabolic, neurological, and ophthalmic disorders. Emerging therapies and research investments are increasing demand for these biologics.
By End User
- Contract Manufacturing Organizations (CMOs)
CMOs are being increasingly preferred due to outsourcing trends, cost savings, and manufacturing expertise. Their importance cannot be overstated in the context of scalability, regulatory compliance and time-to-market for biologics.
- Pharmaceutical & Biotechnology Companies
Pharma and biotech companies continue to be the main end-users, driving innovation and commercialization. Spending on in-house development capabilities for high-value biologics ensures strategic control over key processes and IP.
- Research Institutes & Academic Centers
Research institutes are engaged in early-stage biologics development, new therapies and process development. Partnerships and funding opportunities facilitate translation from research to clinical and commercial development.
Regional Insights
North America, including the United States, Canada, and Mexico, is the leading market for biologics manufacturing, with strong R&D facilities, high regulatory support, and widespread use of advanced therapies. The United States is the leading market for biologics in oncology and infectious diseases, while Canada and Mexico are represented by specialized vaccine and recombinant protein manufacturing. Europe, including Germany, United Kingdom, France, Spain, Italy, and the rest of Europe, has an established biologics manufacturing industry. Germany and the U.K. are the leading countries for monoclonal antibody and cell therapy manufacturing, while France, Italy, and Spain are represented by biosimilar and vaccine development. The region is focused on advanced purification technologies and quality-compliant manufacturing processes.
Asia Pacific, including Japan, China, Australia & New Zealand, South Korea, India, and the rest of Asia Pacific, is the fastest-growing market for biologics manufacturing. Japan and China are the leading countries for monoclonal antibody and vaccine manufacturing, while India and South Korea are expanding their CMO offerings and recombinant protein manufacturing.
The development of new facilities and government initiatives improve capacity and uptake of advanced therapies. South America, including Brazil, Argentina, and the other countries in South America, is slowly developing biologics capacity with emphasis on vaccines and recombinant proteins. The Middle East & Africa, including Saudi Arabia, UAE, South Africa, and other countries, is developing with selective uptake of vaccines and specialized biologics, facilitated by government and international partnerships.
To learn more about this report, Download Free Sample Report
Recent Development News
- June 2025, WuXi Biologics recently announced the initiation of construction for a large-scale microbial manufacturing facility in the Wenjiang district of Chengdu. The new facility will feature a 15,000 L fermenter, a dual-chamber lyophilization line, and vial filling lines with capacity over 10 million annual doses. The new facility will enhance the company’s commercial biologics manufacturing capabilities for peptides, plasmid DNA, enzymes, antibody fragments, cytokines, and VLPs, with GMP launch scheduled for late 2026.
- In October 2024, Samsung Biologics made a big announcement about a large contract manufacturing agreement with a pharmaceutical company based in Asia, which is the largest single client contract in the company’s history, for the production of biologics at its facility in Songdo, South Korea. The agreement is until December 2037 and represents a substantial increase in the biomanufacturing order book of Samsung Biologics, while the company also has expansion plans in the region with offices in the U.S. and Japan.
|
Report Metrics |
Details |
|
Market size value in 2025 |
USD 365.00 Billion |
|
Market size value in 2026 |
USD 385.00 Billion |
|
Revenue forecast in 2033 |
USD 650.00 Billion |
|
Growth rate |
CAGR of 7.80% from 2026 to 2033 |
|
Base year |
2025 |
|
Historical data |
2021 – 2024 |
|
Forecast period |
2026 – 2033 |
|
Report coverage |
Revenue forecast, competitive landscape, growth factors, and trends |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
United States; Canada; Mexico; United Kingdom; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; United Arab Emirates |
|
Key company profiled |
Lonza Group Ltd., Samsung Biologics, WuXi Biologics Cayman Inc., Thermo Fisher Scientific Inc., Catalent, Inc., Boehringer Ingelheim BioXcellence, AGC Biologics, FUJIFILM Diosynth Biotechnologies, Rentschler Biopharma SE, Bioreliance (Merck KGaA), Eurofins Scientific, 3P Biopharmaceuticals, Binex Co. Ltd., Recipharm AB, and Celltrion |
|
Customization scope |
Free report customization (country, regional & segment scope). Avail customized purchase options to meet your exact research needs. |
|
Report Segmentation |
By Product Type (Monoclonal Antibodies, Recombinant Proteins, Vaccines, Cell Therapy Products, Gene Therapy Products, Others), By Technology (Cell Culture, Microbial Fermentation, Chromatography, Filtration, Purification), Additive Manufacturing, Powder Forging, Others), By Application (Oncology, Infectious Diseases, Autoimmune Disorders, Cardiovascular Diseases, Rare Diseases, Others) and By End User (Contract Manufacturing Organizations (CMOs), Pharmaceutical & Biotechnology Companies, Research Institutes & Academic Centers) |
Key Biologics Manufacturing Company Insights
GKN Biologics Manufacturing is a world leader in the Biologics Manufacturing industry, integrating advanced sintered component manufacturing with innovative additive manufacturing powders for the automotive, aerospace, and industrial sectors. Building on decades of expertise in the industry, the company’s diverse product offering encompasses high-performance steel powders, magnetic materials, and specialty alloys developed to satisfy rigorous OEM performance standards. The company’s strategic focus on e-drive powder production capacity and collaboration with additive manufacturing strengthens competitive positioning, particularly in the EV market, while regional diversification underpins solid market presence in North America, Europe, and Asia.
Key Biologics Manufacturing Companies:
- Lonza Group Ltd.
- Samsung Biologics
- WuXi Biologics Cayman Inc.
- Thermo Fisher Scientific Inc.
- Catalent, Inc.
- Boehringer Ingelheim BioXcellence
- AGC Biologics
- FUJIFILM Diosynth Biotechnologies
- Rentschler Biopharma SE
- Bioreliance (Merck KGaA)
- Eurofins Scientific
- 3P Biopharmaceuticals
- Binex Co. Ltd.
- Recipharm AB
- Celltrion
Global Biologics Manufacturing Market Report Segmentation
By Product Type
- Monoclonal Antibodies
- Recombinant Proteins
- Vaccines
- Cell Therapy Products
- Gene Therapy Products
- Others
By Technology
- Cell Culture
- Microbial Fermentation
- Chromatography
- Filtration
- Purification
By Application
- Oncology
- Infectious Diseases
- Autoimmune Disorders
- Cardiovascular Diseases
- Rare Diseases
- Others
By End User
- Contract Manufacturing Organizations (CMOs)
- Pharmaceutical & Biotechnology Companies
- Research Institutes & Academic Centers
Regional Outlook
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- Japan
- China
- Australia & New Zealand
- South Korea
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
- Rest of the Middle East & Africa










 APAC:+91 7666513636
APAC:+91 7666513636





