Market Summary
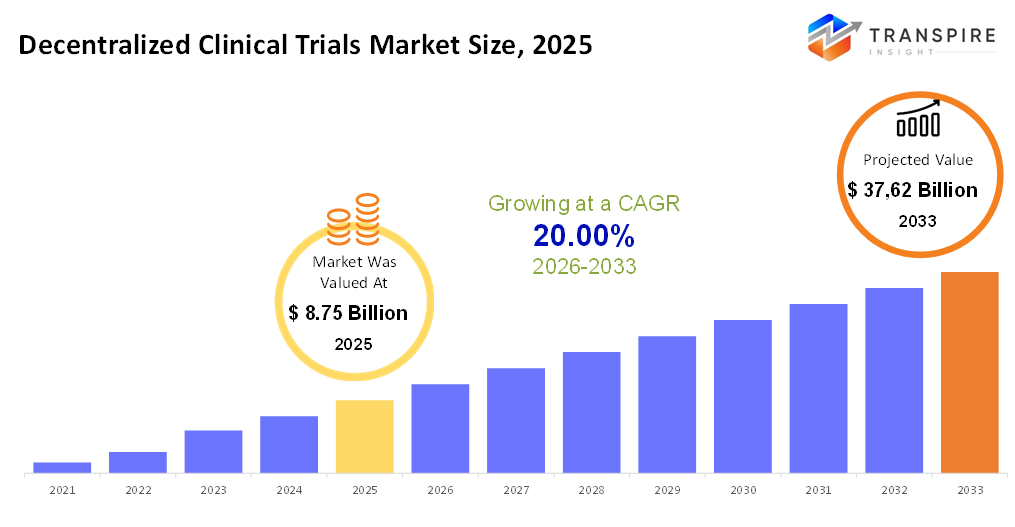
The global Decentralized Clinical Trials market size was valued at USD 8.75 billion in 2025 and is projected to reach USD 37.62 billion by 2033, growing at a CAGR of 20.00% from 2026 to 2033. The decentralized clinical trials approach is gradually being adopted for various stages of clinical research as it allows for better patient data collection, observation, and analysis. Also, patient behavioral and response studies become more accurate with the use of digital technologies in the form of devices and remote monitoring systems, which, in a way, are simplifying the processes and making them faster, thus making this approach more favorable for investors and pharmaceutical companies. On the other hand, the growing prevalence of complex and rare disorders, clinical trials, and R&D spending is opening wide growth avenues in the decentralized clinical trials market, which is a major trend being witnessed in this market.
Market Size & Forecast
- 2025 Market Size: USD 8.75 Billion
- 2033 Projected Market Size: USD 37.62 Billion
- CAGR (2026-2033): 20.00%
- North America: Largest Market in 2026
- Asia Pacific: Fastest Growing Market
To learn more about this report, Download Free Sample Report
Key Market Trends Analysis
- The market of decentralized clinical trials is dominated by North America because it possesses an advanced healthcare infrastructure, has a strong presence of pharmaceuticals and biotechnology firms, and is one of the early adopters of digital health technologies.
- The US is the absolute leader in the North American market, as pharmaceutical companies and CROs are investing heavily in virtual and hybrid trial designs. Supportive regulatory guidance, advanced digital platforms, and access to large, diverse patient populations accelerate the broad-based adoption of decentralized clinical trials across all therapeutic areas.
- The markets in the Asia Pacific are growing very rapidly, powered by expanding clinical research activity, increasing government support for digital healthcare, and growing adoption of remote monitoring technologies in countries such as China, Japan, South Korea, and India.
- A significantly large portion of the market is contributed to interventional trials due to their integral importance in obtaining regulatory approval and drug development. Remote patient monitoring and electronic data capture have significantly enhanced efficiency and patient accrual and retention in interventional trials.
In addition, the market for decentralized clinical trials is growing rapidly as there is an increasing focus on adopting health technologies and patient-centric trial types, and there is an increasing need for improving the efficiency of clinical trials. The market for decentralized clinical trials is led by North America, and the Asia Pacific market is also growing rapidly as there is an increasing focus on improving the infrastructure for clinical trials and there is an increasing potential for costs savings. The increasing focus on interventional trials is an indicator of increasing importance of the role of decentralized trials for drug development and regulatory approval.
Decentralized Clinical Trials Market Segmentation
By Type
- Interventional
Interventional decentralized trials actively test new treatments or interventions while using remote monitoring, telemedicine, and digital tools to reduce site visits and improve patient participation.
- Observational
Observational decentralized trials focus on collecting real-world data from patients in their natural settings using wearable devices, mobile apps, and electronic health records without altering standard care.
- Expanded Access
Expanded access decentralized trials allow patients with serious or life-threatening conditions to receive investigational treatments outside traditional trials, supported by remote follow-ups and digital data collection.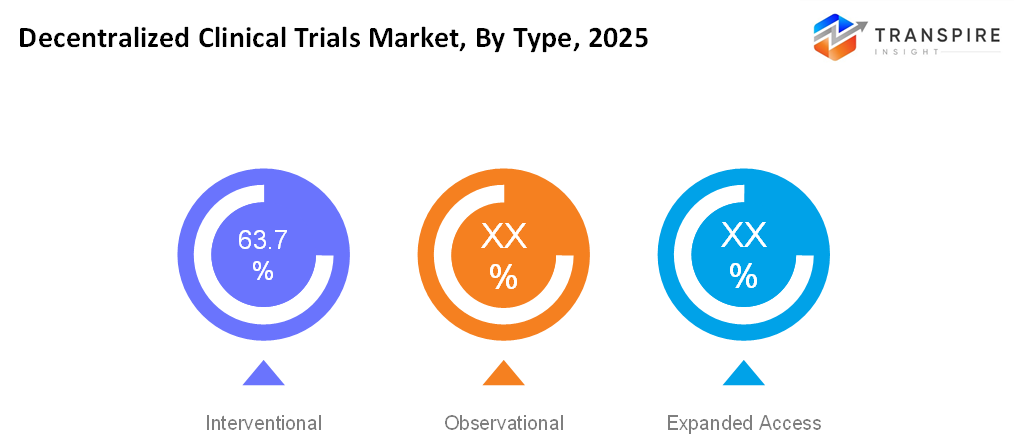
To learn more about this report, Download Free Sample Report
By Therapeutic Application
- Oncology
Oncology is the most popular setting for decentralized trials because of the high number of trials that are complicated and of long duration, and where remote monitoring of trials helps increase patient enrollment and avoids dropouts.
- Cardiology
In cardiology, decentralized clinical trials leverage wearable technology, which allows for continuous observation of vital signs and cardiac functions, thus requiring fewer site visits.
- Neurology
In neurology trials, decentralized studies are advantageous because this allows remote evaluation for symptoms and disease progression, which will be very useful for subjects with mobility or cognitive impairments because they will not need to physically go to the location where the study will be
- Respiratory
In respiratory, one of the advantages of decentralized clinical trials is the ability to monitor respiratory function and symptoms in real time with digital technology.
- Other
This includes other therapeutic areas like endocrinology and rare diseases, where decentralized studies can help serve a more geographically dispersed patient population more effectively.
Regional Insights
Decentralized Clinical Trials Market has a diverse trend in different regions, based on the variability in the availability of healthcare infrastructure, adoption levels in the healthcare sector, government support, investment in research, among others. North America is the largest market, due to the presence of an encouraging pharmaceuticals and biotech sector, huge investment in R&D, as well as robust healthcare IT infrastructure, led by the United States.
Europe comes next with consistent growth due to favorable legal environments, well-structured public healthcare systems, and the adoption of patient-centric and electronic clinical trial solutions in the Western European nations. The Asia-Pacific region is progressively expanding with support from governments for digital health programs, increased research activities, low-cost clinical trial delivery, and development of pharmaceutical and biotech facilities in nations such as China, Japan, South Korea, and India. South America is still an emerging region with Brazil and Argentina advancing with enhanced research facilities and the involvement of nations worldwide in clinical trials. The Middle Eastern and African nations are still in the budding phase with initial adoption by nations that are progressing with healthcare upgrades and digital transformation in healthcare delivery services.
To learn more about this report, Download Free Sample Report
Recent Development News
- In August 2024, the industry-leading clinical trial technology platform, Medable Inc., today unveiled Medable Studio, an all-in-one tool for setting up, translating, validating, and introducing eCOA Plus (eCOA, eConsent, Televisit, and Sensors) into clinical trials.
- In July 2024, Walgreens will receive $25 million in Project NextGen money from the Administration for Strategic Preparedness and Response's Biomedical Advanced Research and Development Authority (BARDA) to undertake a decentralized clinical trial, with the goal of expanding access to clinical trials.
|
Report Metrics |
Details |
|
Market size value in 2025 |
USD 8.75 Billion |
|
Market size value in 2026 |
USD 10.50 Billion |
|
Revenue forecast in 2033 |
USD 37.62 Billion |
|
Growth rate |
CAGR of 20.00% from 2026 to 2033 |
|
Base year |
2025 |
|
Historical data |
2021 – 2024 |
|
Forecast period |
2026 – 2033 |
|
Report coverage |
Revenue forecast, competitive landscape, growth factors, and trends |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
United States; Canada; Mexico; United Kingdom; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; United Arab Emirates |
|
Key company profiled |
IQVIA, Labcorp, ICON, Covance, CRF Health, Parexel, LEO Innovation Lab, Thermo Fisher, Huma, Oracle, Medidata, PRA Health Sciences, Clinical Ink, Signant Health, Medable, and Science 37 |
|
Customization scope |
Free report customization (country, regional & segment scope). Avail customized purchase options to meet your exact research needs. |
|
Report Segmentation |
By Type (Interventional, Observational, Expanded Access), By Therapeutic Application (Oncology, Cardiology, Neurology, Respiratory, and Other) |
Key Decentralized Clinical Trials Company Insights
With its broad portfolio-including IoT-enabled precision equipment, autonomous machinery, and data analytics platforms-Deere & Company is a clear market leader. Its global presence and significant investment in research and development allow the company to continue to innovate, providing scalable solutions for farmers that help improve operational efficiency and sustainability. Deere's ability to integrate hardware and software ecosystems provides a competitive advantage, which fosters wide diffusion across large commercial farms worldwide.
Key Decentralized Clinical Trials Companies:
- IQVIA
- Labcorp
- ICON
- Covance
- CRF Health
- Parexel
- LEO Innovation Lab
- Thermo Fisher
- Huma
- Oracle
- Medidata
- PRA Health Sciences
- Clinical Ink
- Signant Health
- Medable
- Science 37
Global Decentralized Clinical Trials Market Report Segmentation
By Type
- Interventional
- Observational
- Expanded Access
By Therapeutic Application
- Oncology
- Cardiology
- Neurology
- Respiratory
- Other
Regional Outlook
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- Japan
- China
- Australia & New Zealand
- South Korea
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
- Rest of the Middle East & Africa










 APAC:+91 7666513636
APAC:+91 7666513636





