
Jan 09, 2026
The report “Continuous Manufacturing in Pharma Market By Product (APIs, Finished Dosage Forms), By Technology (Continuous Flow Reactors, Process Analytical Technology), By Drug Type (Small Molecule Drugs, Biologics & Vaccines)” is expected to reach USD 10.65 billion by 2033, registering a CAGR of 16.80% from 2026 to 2033, according to a new report by Transpire Insight.
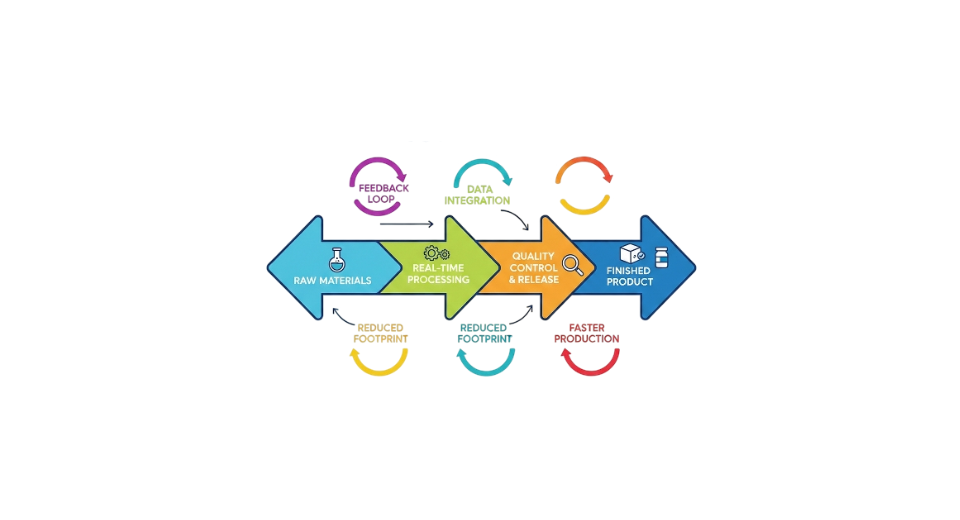
The pharmaceutical companies are changing the way they make medicines. They are moving away from the way of making medicines in batches to a new way that is continuous and streamlined. This new way of making medicines is better because it helps make the active ingredients and the final medicines faster and cheaper. Companies can also make sure the medicines are of quality. The pharmaceutical companies want to get the medicines to the people who need them faster. They also want to make sure the medicines are safe for the patients and that they are all the quality. That is why they are starting to use the manufacturing method all over the world. The continuous manufacturing in pharma market is growing because of this change. Pharmaceutical companies, like this way of making medicines because it saves them time and money.
Geographically, North America is the leader when it comes to the market. This is because North America has support from the government and a good system in place for making things. North America also started using ways of making things very early.Europe is behind North America. This is happening because Europe really cares about quality and new ideas in countries like Germany, the UK and France.
The market is further fueled by growing global pharmaceutical demand, expansion of production capacities, and increasing collaboration between pharmaceutical companies, technology providers, and CDMOs. Companies are focusing on scalable, cost-efficient solutions to address supply chain challenges and regulatory requirements. With advancements in automation, digital monitoring, and integration of AI-based process optimization, continuous manufacturing is positioned as a strategic approach for efficient, high-quality, and sustainable pharmaceutical production in the coming years.
The finished dosage forms segment is projected to witness the highest CAGR in the Continuous Manufacturing in Pharma market during the forecast period.
According to Transpire Insight, finished dosage forms are the important part of the product category. This is because people want medications that're ready for patients to use and that are always of good quality and safe. Finished dosage forms are different from Active Pharmaceutical Ingredients. Active Pharmaceutical Ingredients are mostly about the chemical in medicine. Finished dosage forms include things, like tablets, capsules and liquids that people can take. When we make these finished dosage forms continuously, we can watch what is happening at every step make sure the dosage is just right and that every product is the same. This helps reduce mistakes and makes sure we follow the rules that regulators set for dosage forms. Pharmaceutical companies are investing in dedicated continuous lines for FDFs, driven by rising global demand and the need to optimize production efficiency. This segment benefits from automation and advanced process control, which support scalability and reduce operational costs.
The use of manufacturing for finished dosage forms is really popular in North America and Europe. This is because the people in charge of regulations in these places want companies to use better ways of making drugs. They think this will make the drugs better and more reliable.
In Asia Pacific things are changing fast. Countries like India, China and Japan are building advanced facilities to make more drugs for their own people and to sell to other countries. Continuous manufacturing is getting a boost from new technologies. For example, there is something called process analytical technology or PAT, for short and hybrid batch-continuous systems. These things help companies make a lot of drugs quickly while saving time. Continuous manufacturing is growing because of these technologies. These factors collectively finished dosage forms as the largest and most strategically significant product segment in the market.
The Continuous flow reactors segment is projected to witness the highest CAGR in the Continuous Manufacturing in Pharma market during the forecast period.
Continuous flow reactors are good at helping us make chemicals. They make the process of making these chemicals more efficient. They give us more of the chemical we want. This is because we can control things like how hot or cold it's how much pressure is used and how long the chemical is being made. This means we get a product.
Pharmaceutical manufacturers like flow reactors when they are making small molecule drugs. This is because continuous flow reactors help reduce the differences, we see from one batch to another. They also help us use the materials we need more efficiently, and they reduce the amount of waste we make. Continuous flow reactors are very useful to produce APIs and intermediates. These machines are made to fit into the production lines that companies already have. This means that companies can use these machines for test runs or for making large quantities of products. The modular design of these machines provides flexibility, for both scale and large-scale manufacturing.
The use of flow reactors is being pushed forward by regulatory agencies in North America and Europe. They want to use technologies that make the process more reliable and reduce the risk of batch failures. Continuous flow reactors are also getting attention in Asia Pacific. This is because people in Asia Pacific want to make cost- high-quality pharmaceutical products. Continuous flow reactors are helpful because they support process analytical technology integration. This means we can watch what is happening in time and make changes as we go. This makes the whole process more efficient and compliant. Continuous flow reactors are a part of this. The combination of operational efficiency, scalability, and regulatory alignment makes continuous flow reactors to the leading technology segment in the market.
The Small molecule drugs segment is projected to witness the highest CAGR in the Continuous Manufacturing in Pharma market during the forecast period.
According to Transpire Insight, Small molecule drugs are popular when it comes to the type of drugs that are made. This is because they are simpler to make and a lot of people need them. They also work well with machines that can make drugs all the time. Small molecule drugs make up most of the medicines that are made around the world. So, it makes sense that they are a choice, for being made in a continuous process. Making drugs in a process is good because it does not take as long and the quality is always the same. It also helps the people who make the drugs because they can make more when they need to.
The people who make molecule drugs like to use special machines that help them make the drugs and check their quality at the same time. These machines are called flow reactors and PAT systems. They help with making the drugs getting them ready to use and checking to make sure they are good. All these steps can be done in one process, which makes things easier. The market for molecule drugs is doing well in North America. This is where big pharmaceutical companies are using a way of making drugs to get more work done and follow the rules. North America is a place for small molecule drugs.
Europe is also doing a job with small molecule drugs. Countries like Germany, the UK and France are really into making sure everyone follows the rules and tries things. Small molecule drugs are also getting popular in Asia Pacific. India, China and Japan are building factories to make small molecule drugs for people in their own countries and for other countries too. Small molecule drugs are a deal, in these places. The combination of technological adaptability, cost efficiency, and regulatory alignment positions small molecule drugs as the top-performing drug type segment in continuous manufacturing.
The North America region is projected to witness the highest CAGR in the Continuous Manufacturing in Pharma market during the forecast period.
North America is the leader when it comes to manufacturing in the pharma market. This is because North America has an established pharmaceutical system, good manufacturing infrastructure and rules that help things move forward. The United States is the part of North America in this area. The United States has seen a lot of companies use manufacturing technologies to make APIs and finished dosage forms. This is happening because the FDA is supporting ways of doing things and wants companies to monitor quality in real time. The FDA and continuous manufacturing in pharma are important, for North America and the United States. Companies are putting a lot of money into flow reactors and modular systems. They also use process technology, which is also known as PAT. The reason they do this is to make their work more efficient, to make products faster and to make sure the products are always good. This area is good at this because there are big pharmaceutical companies and CDMOs here. These companies like to use automation and digital integration. They want to be able to make a lot of products. This helps them to meet the needs of patients and to supply the world with the products they need. Companies like flow reactors because they help with production.
North America has a strong position in the market. This is because they spend a lot of money on research and development and they are always trying out technologies. The big pharmaceutical companies in North America use something called manufacturing. This helps them make sure that every batch of medicine is the same, that they do not waste any materials and that their machines work properly all the time. Canada and Mexico are also becoming places for making medicines. They offer a lot of flexibility. Can help keep costs down. North America is a place for continuous manufacturing because the government helps there are a lot of smart people who know about technology and the roads and buildings are all, in good shape. All of this means that North America can make medicines that're good quality that are made efficiently and that follow all the rules. North America and its continuous manufacturing are a combination. This positions the region not only as the current leader but also as a benchmark for global adoption of advanced pharmaceutical manufacturing solutions.
Key Players
The top 15 players in the Continuous Manufacturing in Pharma market include GEA Group AG, Thermo Fisher Scientific Inc., Siemens AG, Glatt GmbH, L.B. Bohle Maschinen + Verfahren GmbH, Coperion GmbH, Scott Equipment Company, Syntegon Technology, Continuus Pharmaceuticals, Baker Perkins Ltd., Fette Compacting GmbH, Gericke AG, Hosokawa Micron Corporation, Chemtrix BV, Corning Inc.
Drop us an email at:
Call us on:
+91 7666513636