Market Summary
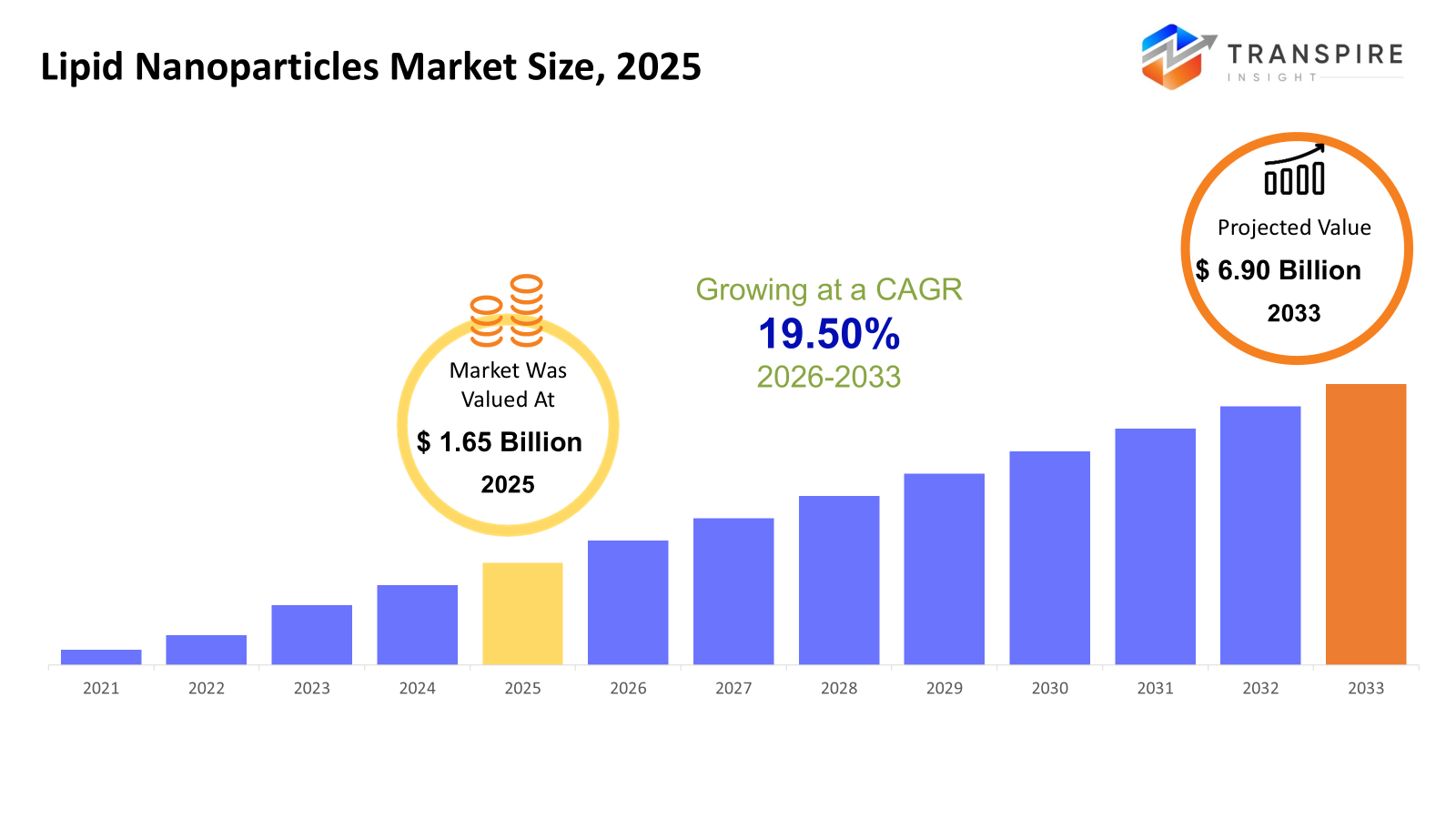
The global Lipid Nanoparticles market size was valued at USD 1.65 billion in 2025 and is projected to reach USD 6.90 billion by 2033, growing at a CAGR of 19.50% from 2026 to 2033. The market for lipid nanoparticles is growing rapidly due to the increasing use of RNA-based treatments, such as gene therapies and mRNA vaccinations, where lipid nanoparticles facilitate the safe and effective delivery of nucleic acids. Commercialization operations throughout pharmaceutical pipelines are accelerating due to increased investment in biologics development and nanomedicine research. The market is expanding due to the growing need for targeted drug delivery methods that increase efficacy while lowering systemic toxicity. Furthermore, improved production efficiency and wider clinical usage are made possible by technological developments in formulation and scalable manufacturing techniques.
Market Size & Forecast
- 2025 Market Size: USD 5.10 Billion
- 2033 Projected Market Size: USD 6.90 Billion
- CAGR (2026-2033): 19.50%
- North America: Largest Market in 2026
- Asia Pacific: Fastest Growing Market
To learn more about this report, Download Free Sample Report
Key Market Trends Analysis
- North America continues to maintain leadership due to advanced biopharmaceutical infrastructure, strong clinical research activity, and early adoption of RNA-based therapeutics, supported by regulatory support and increasing commercialization of lipid nanoparticle-enabled vaccines and targeted treatment platforms across healthcare systems.
- Investing heavily in biotechnology, having a large number of top pharmaceutical companies, and conducting more clinical trials for gene therapy and oncology applications, the US propels regional innovation and speeds up the adoption of lipid nanoparticle technologies in both research and commercial manufacturing settings.
- Growing pharmaceutical production in China, Japan, South Korea, and India, increased government support for nanotechnology research, and expanding biotechnology manufacturing capacity are all contributing to Asia Pacific's accelerated growth and the increasing use of lipid nanoparticle-based drug delivery and vaccine development platforms.
- Liposomes remain the leading type segment due to established clinical validation, regulatory familiarity, and broad applicability across oncology and vaccine delivery, while nanostructured lipid carriers are gaining traction due to higher drug loading efficiency and improved stability for advanced therapeutic applications.
- Lipid nanoparticles greatly increase bioavailability and allow for the targeted delivery of complex therapeutics, making drug delivery the most popular application segment. Meanwhile, vaccine development is still growing, with a greater emphasis on mRNA platforms and rapid-response infectious disease treatment strategies worldwide.
- Microfluidic technology can guarantee accurate particle size control, reproducibility and scalability it is becoming the preferred manufacturing method for RNA therapeutics, supporting large scale production requirements and improving consistency in pharmaceutical-grade lipid nanoparticle formulations.
- The main end users are pharmaceutical and biotechnology firms, which are motivated by rising research expenditures, growing biologics pipelines, and an increasing dependence on lipid nanoparticle platforms to facilitate the commercialization of gene therapies, vaccines and precision medicine applications in international healthcare markets.
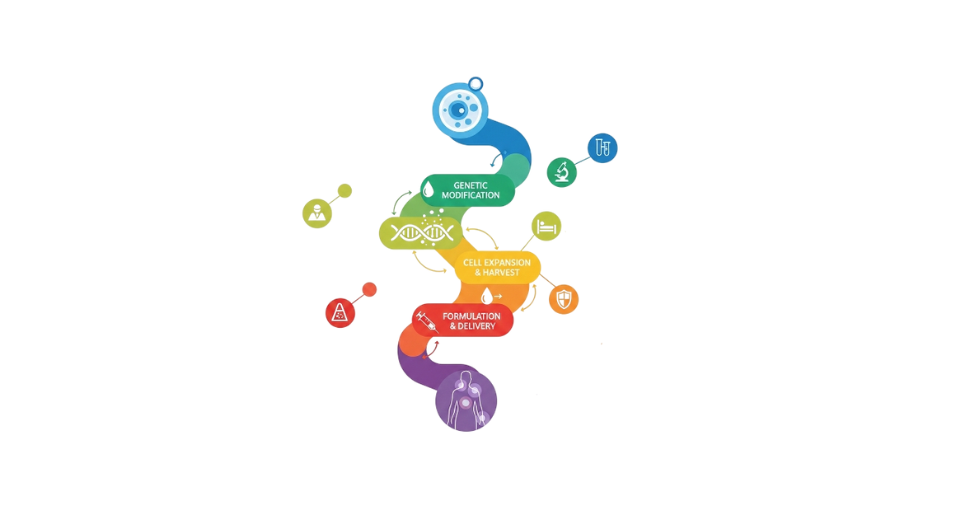
So, The development and marketing of lipid-based nanoscale delivery systems intended to encapsulate and transport medicinal molecules, such as medications, nucleic acids, and biologics, is referred to as the lipid nanoparticles market. These nanoparticles are crucial in contemporary pharmaceutical and biotechnology applications because they improve medication stability, bioavailability, and targeted administration. Lipid nanoparticles are becoming an essential aspect of sophisticated medication delivery systems due to their capacity to shield delicate molecules from deterioration. The market has gained significant momentum following the successful deployment of lipid nanoparticles in mRNA vaccine platforms, which demonstrated their effectiveness in delivering genetic material safely and efficiently. Application fields are being expanded by growing research in oncology, gene therapy, and the treatment of rare diseases. Lipid nanoparticle technologies are being included into research pipelines by pharmaceutical companies more frequently in an effort to enhance therapeutic outcomes and lessen side effects. Technological developments in production scalability and formulation techniques are bolstering market expansion. New therapeutic opportunities are being made possible by advancements in lipid chemistry, particle engineering and controlled release mechanisms. Lipid nanoparticles are anticipated to continue to be a fundamental technology supporting next-generation pharmaceutical innovation as precision medicine and biologics continue to grow internationally.
Lipid Nanoparticles Market Segmentation
By Type
- Solid Lipid Nanoparticles (SLNs)
Due to excellent physical stability and regulated drug release capabilities, solid lipid nanoparticles are a well-established delivery system. They reduce the risk of toxicity while improving the bioavailability of poorly soluble medications. Sensitive medicinal molecules are protected during circulation by their solid lipid matrix. Consistent market demand is supported by growing use in dermatological and pharmaceutical formulations.
- Nanostructured Lipid Carriers (NLCs)
Nanostructured Lipid Carriers (NLCs) have a higher drug loading capacity and less drug ejection than conventional SLNs, nanostructured lipid carriers are becoming more and more popular. Their hybrid lipid composition allows for versatile formulation design and improves stability. NLCs are being investigated extensively for enhanced medicinal applications and nucleic acid delivery. Adoption is speeding up due to increased research in targeted medicine delivery.
- Liposomes
One of the most developed lipid nanoparticle technologies for commercial use, liposomes are frequently utilized in authorized medication compositions. Their bilayer shape improves therapeutic efficacy by enabling the encapsulation of both lipophilic and hydrophilic medications. Continued commercial expansion is supported by robust clinical validation and regulatory acceptability. Growth is sustained by expanding use in vaccine administration and oncology.
- Lipid–Polymer Hybrid Nanoparticles
Lipid-polymer hybrid nanoparticles combine the biocompatibility of lipids with the structural strength of polymers. Drug stability, controlled release, and targeted effectiveness are all enhanced by this hybrid architecture. Applications for precision medicine and gene delivery are expanding in this market. Nanomedicine's clinical potential is growing due to ongoing innovation.
- Cationic Lipid Nanoparticles
Cationic lipid nanoparticles may bind negatively charged RNA and DNA molecules, they are essential for the delivery of nucleic acids. They are widely employed in the development of gene therapy and mRNA vaccinations. To address toxicity concerns, however, formulation adjustment is necessary. Safety and efficacy profiles are being improved by ongoing developments in lipid chemistry.
- PEGylated Lipid Nanoparticles
PEGylated lipid nanoparticles are intended to decrease immune system recognition and increase circulation time. In systemic administration, PEG modification improves pharmacokinetics and boosts therapeutic stability. Advanced biologics and RNA-based treatments make extensive use of these nanoparticles. The industry is expanding due to the growing need for long-acting medication delivery methods.
- Others
Other types of lipid nanoparticles include newly developed lipid formulations and tailored delivery systems. These systems are frequently tailored to particular applications and therapeutic needs. Innovation in this field is influenced by ongoing research in lipid chemistry and nanocarrier engineering. The category shows how nanoparticle technologies continue to diversify.
By Application
- Drug Delivery
Lipid nanoparticles can improve drug solubility, stability and targeted distribution, drug delivery is the most popular application area. They allow for better therapeutic results and fewer doses. Sustained acceptance is supported by the growing development of biologics and precision medicines. Pharmaceutical firms are still making significant investments in LNP-based delivery systems.
- Vaccine Development
The use of lipid nanoparticles in mRNA vaccine delivery, which facilitates effective cellular absorption and antigen expression has drawn attention from all around the world. The technology facilitates scalable production and quick vaccine development. Demand is being driven by ongoing research on infectious illnesses and customized vaccines. This market is further strengthened by the growth of RNA-based treatments.
- Gene therapy
Lipid nanoparticles provide safe and effective nucleic acid transport without the need for viral vectors in gene therapy applications. When compared to conventional methods, LNPs offer decreased immunogenicity and increased delivery efficiency. Market potential are growing as a result of an increase in clinical trials for rare diseases and genetic abnormalities. Prospects for commercialization are also being supported by regulatory advancements.
- Cancer Therapy
Lipid nanoparticles minimize systemic toxicity and provide tailored medication delivery in oncology. They make it easier for chemotherapeutics, RNA therapies, and immunomodulatory drugs to be delivered. Adoption is speeding up due to rising demand for precision oncology therapies. Pipeline development is being strengthened by research partnerships between biotechnology companies and academic institutions.
- Diagnostics
Applications for biomarker detection and diagnostic imaging are using lipid nanoparticles more frequently. Their capacity to transport imaging materials enhances disease detection's sensitivity and specificity. Adoption is being aided by advancements in early disease diagnosis and individualized care. Although the market is still focused on research, it has a lot of long-term promise.
- Cosmetics & Dermatology
- Lipid nanoparticles improve ingredient stability and penetration in topical formulations used in dermatology and cosmetics. They make better skin compatibility and controlled release possible. Growing consumer demand for cutting-edge skincare products encourages market growth. The growing use of nanotechnology in personal care products is advantageous to this market.
- Nutraceuticals
Vitamins, antioxidants, and nutraceutical substances can have their bioavailability increased by using lipid nanoparticles. Encapsulation improves absorption and shields active substances from deterioration. Demand is supported by rising consumer awareness of functional nutrition. Oral delivery system innovation is a factor in the segment's expansion.
- Scientific Research
Applications of scientific research are still crucial for the advancement of technology and the approval of novel lipid formulations. LNPs are used in research labs and academic institutions for experimental medication and gene delivery experiments. Sustained demand is supported by rising investments in biomedical and nanotechnology research. Future commercialization relies heavily on this segment.
By Technology
- Microfluidic Technology
When producing lipid nanoparticles, microfluidic technology allows for exact control over particle size, content, and reproducibility. It facilitates scalable production appropriate for both commercial and medical uses. For RNA-based treatments, the technology is becoming more and more popular. Adoption is being driven by the growing need for standardized industrial procedures.
- Ethanol Injection
For the creation of nanoparticles at the laboratory scale, ethanol injection is still a popular and economical technique. It provides ease of use and quick formulation creation. However, its application in large-scale production is constrained by scalability issues. The approach is still crucial for formulation optimization and early-stage research.
- Sonication
Sonication technology reduces particle size and achieves uniform dispersion by using ultrasonic energy. It is frequently employed in small-scale manufacturing and research settings. The method makes it possible to encapsulate medicinal substances effectively. It is still useful for experimental applications despite its scalability limits.
- Jet Homogenization
High-energy mixing made possible by jet homogenization produces tiny nanoparticles with a uniform size distribution. The technique promotes increased encapsulation effectiveness and stability. It is being utilized more and more in manufacturing settings on an industrial scale. Its acceptance is being aided by rising demands for pharmaceutical production.
- High-pressure Homogenization
The durability and scalability of high-pressure homogenization make it a popular method for producing lipid nanoparticles on a large scale. The procedure guarantees improved stability and consistent particle size. For commercial pharmaceutical manufacture, it is especially appropriate. Market expansion is supported by rising demand for LNP-based medicines produced in large quantities.
- Others
Other technologies include proprietary production methods and new formulation processes. These techniques seek to increase encapsulation stability, scalability, and efficiency. The range of production alternatives is increasing due to ongoing advancements in processing technologies. Continuous developments in the production of nanoparticles are reflected in this category.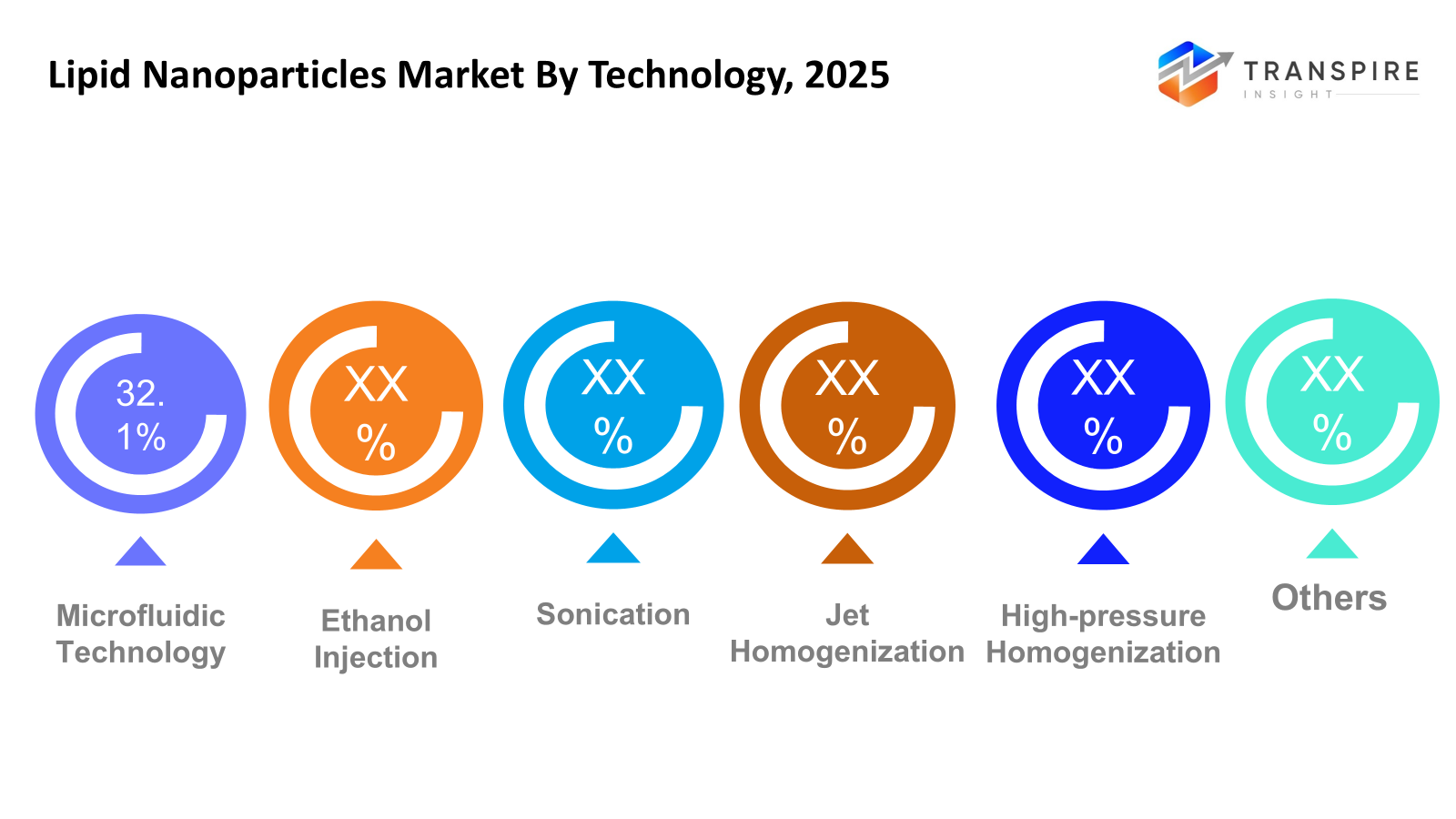
To learn more about this report, Download Free Sample Report
By End User
- Pharmaceutical & Biotechnology Companies
Due to significant investments in RNA therapies and tailored drug delivery systems, pharmaceutical and biotechnology industries comprise the largest end-user segment. LNP technology facilitates pipeline extension in oncology, gene therapy, and vaccines. Adoption is fueled by strong R&D skills and commercialization possibilities. Strategic alliances bolster market expansion even further.
- Academic & Research Institutes
Early-stage innovation and clinical research utilizing lipid nanoparticles are greatly aided by academic and research institutions. These organizations concentrate on developing novel therapeutic applications and optimizing formulations. Adoption is supported by an increase in both public and private research funding. Their contribution to the development of next-generation nanomedicine technology is still crucial.
- Contract Manufacturing Organizations (CMOs/CDMOs)
When it comes to scaling up the production of lipid nanoparticles for clinical trials and commercial supply, CMOs and CDMOs are crucial. Pharmaceutical manufacturing is becoming more dependent on specialized manufacturers due to outsourcing trends. These groups facilitate regulatory compliance and offer technical expertise. The expansion of RNA-based treatments is increasing demand.
- Contract Research Organizations (CROs)
CROs provide pharmaceutical businesses with lipid nanoparticle clinical trial services, formulation evaluation, and preclinical testing. The expansion of this segment is being driven by an increase in the outsourcing of research operations. CROs lower operating expenses and expedite development schedules. Clinical pipeline expansion helps maintain demand.
- Hospitals & Specialty Clinics
Clinical use and sophisticated treatment procedures are the main ways that hospitals and specialty clinics implement lipid nanoparticle-based treatments. Adoption is aided by the growing availability of RNA-based medications and tailored therapy. Demand is being driven by the expansion of specialty treatments and personalized medicine. With more regulatory clearances, the segment is anticipated to grow.
Regional Insights
North America, including the United States, Canada, and Mexico, represents the largest regional market supported by strong biotechnology ecosystems, advanced clinical research infrastructure, and high investment in RNA therapeutics. Canada contributes through academic research and partnerships in biotechnology, while the US dominates in innovation and commercialization. Mexico is assisting the growth of regional supply chains by progressively increasing its capacity to manufacture pharmaceuticals. Europe, which includes Germany, the UK, France, Spain, Italy, and the rest of the continent, shows consistent growth propelled by robust pharmaceutical production and encouraging regulatory environments. France and Italy contribute through biotechnology innovation and clinical development programs, while Germany and the UK spearhead research projects in gene therapy and nanomedicine. Technological advancement is supported by cooperative research funding throughout the region. Due to growing pharmaceutical output and rising government investment in biotechnology, Asia Pacific which includes Japan, China, Australia and New Zealand, South Korea, India, and the rest of Asia Pacific is becoming a high-growth region. While South Korea and India are bolstering their clinical research and contract manufacturing capacities, China and Japan continue to dominate manufacturing and research activity. Through academic and scientific partnerships, Australia and New Zealand foster innovation. Adoption is happening gradually throughout South America, including Brazil, Argentina, and the rest of the region, thanks to expanding pharmaceutical investments and better healthcare infrastructure. Argentina contributes by participating in research, whereas Brazil drives regional demand by growing biotechnology efforts.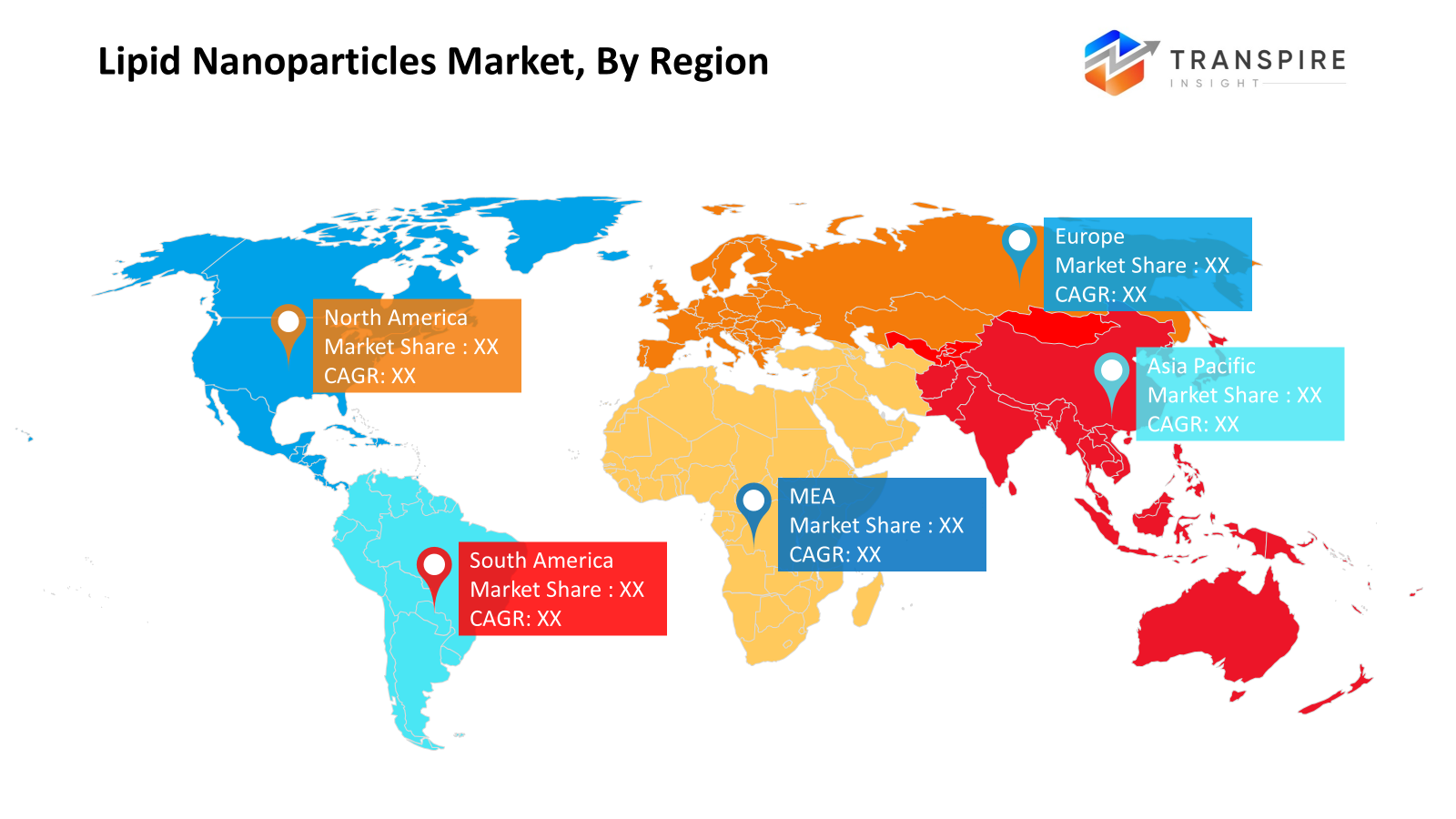
To learn more about this report, Download Free Sample Report
Recent Development News
- In November 2025, Moderna disclosed further investments in its mRNA platform, including advancements in lipid nanoparticle delivery methods to improve therapeutic stability, targeting effectiveness, and scalability. The company emphasized the importance of lipid nanoparticles in mRNA drug delivery innovation and commercialization by highlighting ongoing development initiatives and manufacturing expansion supporting vaccines and medicines made possible by patented LNP systems.
- September 2025, Evonik announced expansion of its lipid-based drug delivery and lipid nanoparticle manufacturing capabilities to support growing demand for mRNA and advanced therapeutics. In order to assist pharmaceutical partners in creating RNA-based medications and vaccines, the company prioritized expanding GMP production and bolstering its formulation and contract development capabilities. The development is a reflection of the growing demand from biotech businesses for outsourcing as well as the commercialization of LNP-based drug delivery platforms.
|
Report Metrics |
Details |
|
Market size value in 2025 |
USD 1.65 Billion |
|
Market size value in 2026 |
USD 2.00 Billion |
|
Revenue forecast in 2033 |
USD 6.90 Billion |
|
Growth rate |
CAGR of 19.95% from 2026 to 2033 |
|
Base year |
2025 |
|
Historical data |
2021 – 2024 |
|
Forecast period |
2026 – 2033 |
|
Report coverage |
Revenue forecast, competitive landscape, growth factors, and trends |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
United States; Canada; Mexico; United Kingdom; Germany; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Argentina; South Africa; Saudi Arabia; United Arab Emirates |
|
Key company profiled |
Moderna, Inc., Pfizer Inc., BioNTech SE, Evonik Industries AG, Merck KGaA, CordenPharma, Precision NanoSystems (Danaher Corporation), Acuitas Therapeutics, Genevant Sciences, Arcturus Therapeutics, Polymun Scientific, Sartorius AG, Croda International plc (Avanti Polar Lipids), Aptar Pharma, Catalent, Inc. (Exelead) |
|
Customization scope |
Free report customization (country, regional & segment scope). Avail customized purchase options to meet your exact research needs. |
|
Report Segmentation |
By Type (Solid Lipid Nanoparticles (SLNs), Nanostructured Lipid Carriers (NLCs), Liposomes, Lipid–Polymer Hybrid Nanoparticles, Cationic Lipid Nanoparticles, PEGylated Lipid Nanoparticles, Others), By Application (Drug Delivery, Vaccine Development, Gene Therapy, Cancer Therapy, Diagnostics, Cosmetics & Dermatology, Nutraceuticals, Scientific Research), By Type (Microfluidic Technology, Ethanol Injection, Sonication, Jet Homogenization, High-pressure Homogenization, Others) and By End User ( Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Contract Manufacturing Organizations (CMOs/CDMOs), Contract Research Organizations (CROs), Hospitals & Specialty Clinics) |
Key Lipid Nanoparticles Company Insights
Moderna, Inc.'s vertically integrated mRNA platform and broad commercialization of LNP-enabled therapies make it a dominant force in the lipid nanoparticles market. The company's success in creating mRNA vaccines strengthened its manufacturing capabilities and technology leadership by expediting the large-scale validation of lipid nanoparticle delivery methods. Moderna's competitive positioning is strengthened by its continuous investments in next-generation LNP formulations, enhanced stability profiles, and increased therapeutic uses in rare illnesses and oncology. While ongoing clinical pipeline expansion supports the ongoing demand for cutting-edge lipid nanoparticle delivery solutions in both preventive and therapeutic applications its robust intellectual property portfolio and international alliances further increase market influence.
Key Lipid Nanoparticles Companies:
- Moderna, Inc.
- Pfizer Inc.
- BioNTech SE
- Evonik Industries AG
- Merck KGaA
- CordenPharma
- Precision NanoSystems (Danaher Corporation)
- Acuitas Therapeutics
- Genevant Sciences
- Arcturus Therapeutics
- Polymun Scientific
- Sartorius AG
- Croda International plc (Avanti Polar Lipids)
- Aptar Pharma
- Catalent, Inc. (Exelead)
Global Lipid Nanoparticles Market Report Segmentation
By Type
- Solid Lipid Nanoparticles (SLNs)
- Nanostructured Lipid Carriers (NLCs)
- Liposomes
- Lipid–Polymer Hybrid Nanoparticles
- Cationic Lipid Nanoparticles
- PEGylated Lipid Nanoparticles
- Others
By Application
- Drug Delivery
- Vaccine Development
- Gene Therapy
- Cancer Therapy
- Diagnostics
- Cosmetics & Dermatology
- Nutraceuticals
- Scientific Research
By Technology
- Microfluidic Technology
- Ethanol Injection
- Sonication
- Jet Homogenization
- High-pressure Homogenization
- Others
By End User
- Pharmaceutical & Biotechnology Companies
- Academic & Research Institutes
- Contract Manufacturing Organizations (CMOs/CDMOs)
- Contract Research Organizations (CROs)
- Hospitals & Specialty Clinics
Regional Outlook
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- Japan
- China
- Australia & New Zealand
- South Korea
- India
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
- Rest of the Middle East & Africa












 APAC:+91 7666513636
APAC:+91 7666513636





